Reduction of Nitro Groups
Reduction of Nitro Groups
The nitro group on benzene ring can be reduced into amino group to form aniline.
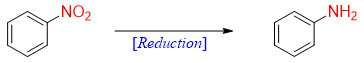
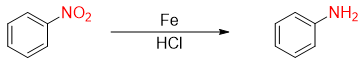
The conversion of nitrobenzene to aniline was first performed by Russian organic chemist Nikolay Zinin in 1842. This reduction reaction was named as Zinin reaction.

A French chemist Antoine Béchamp, developed a method called Bechamp reduction to reduce nitrobenzene to aniline using Fe and HCl.
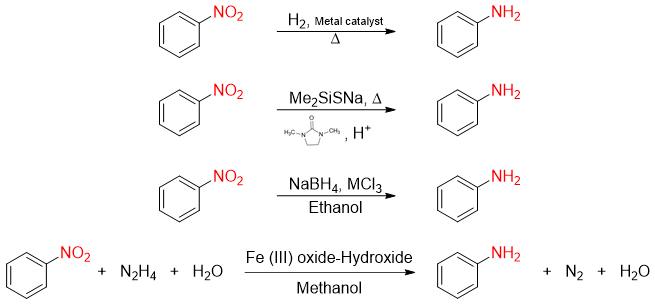
Now a days, the reaction usually performed for the reduction of nitrobenzene to aniline is shown below.
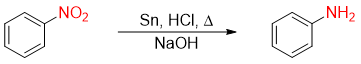
The mechanism of reduction of nitrobenzene is has been assumed to progress with two intermediates shown below.
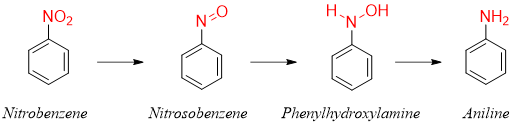
Other reported methods for the conversion of nitrobenzene to aniline are shown below.
Aniline and its derivatives are widely used in the preparation of different useful products including polymers, synthetic dyes, and medicine.
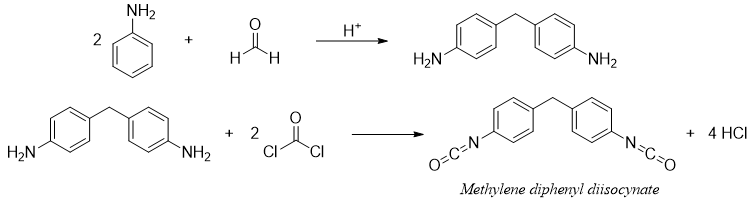
In polymer chemistry, aniline is treated with formaldehyde to produce diamine. Diamine is further treated with phosgene to produce methylene diphenyl diisocynate which is a precursor to urethane polymers.
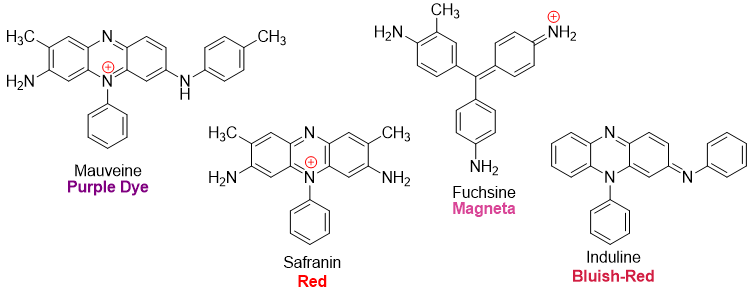
Different synthetic dyes made from aniline are shown below.
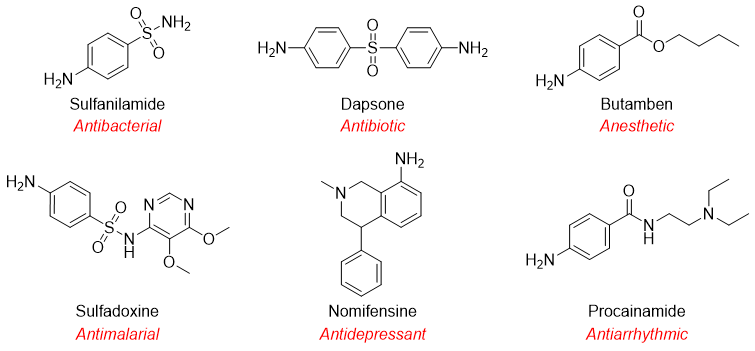
Following are some aniline derived drugs.