(SnAr) Elimination/Addition Reactions
(SnAr) Elimination Addition Reactions
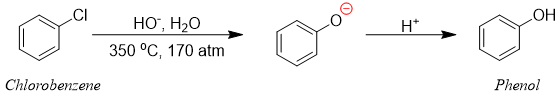
Halobenzenes containing electron withdrawing substituents (EWG) can react with nucleophiles to undergo nucleophilic aromatic substitution reactions while, aromatic rings containing no EWG’s do not show nucleophilic substitution reactions. In 1928, chemists discovered a method in which chlorobenzene was converted to phenol using high temperatures and pressures.
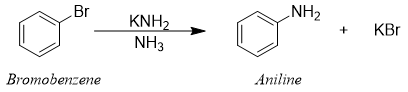
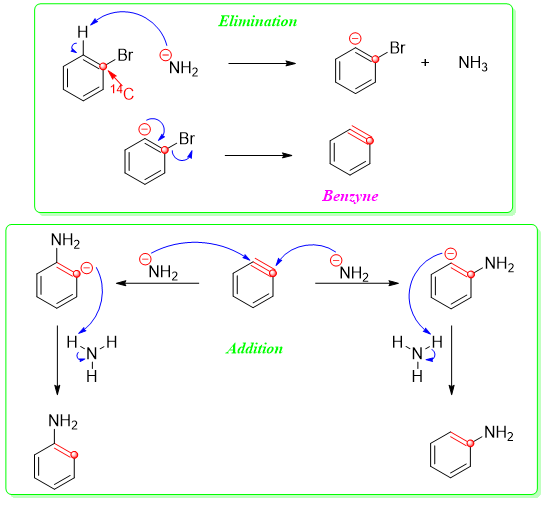
Same type of reaction was observed when bromobenzene was treated with strong base like potassium amide using ammonia as a solvent.
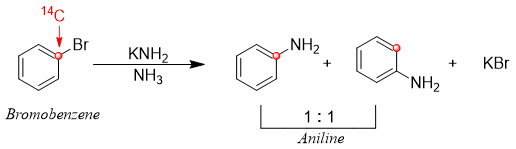
Interesting results were obtained when the C1 carbon of bromobenzene was labeled with radioactive 14C. This labeled bromobenzene gave two products i.e., amine group substituting at position 1 and position 2 in 1:1 ratio. This result suggested the formation of symmetrical intermediate.
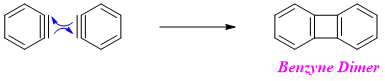
Later mechanistic studies showed that the reactive intermediate formed in this type of reactions is benzyne. Therefore, this type of mechanism is also called as “The Benzyne mechanism”.
Benzyne being too reactive can not be isolated therefore it reacts readily once it is formed. The mechanism of this reaction completes in two steps. The first step is the elimination in which the base abstracts the proton present at position 2. This results in the formation of carbanion which expels bromide to become neutral specie. In the second step, the nucleophile adds to the two carbons forming benzyne in 50:50 ratio.
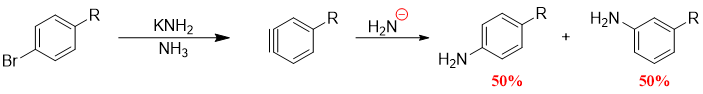
Alkylbenzenes containing halides at para position gives only one benzyne and it gives mixture of products.
The production of mixture of products is not necessary to take place in some of benzene derivatives. Ortho-chloro aryl ethers when treated with sodium amide in liquid ammonia yields single product.
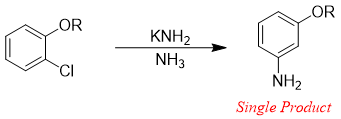
There are two reasons for no formation of ortho product. i) The presence of negative charge (carbanion) next to the oxygen atom (electronegative) is preferred as the oxygen atom withdraws electrons inductively and stabilizes the carbanion. ii) The attack of nucleophile takes place in the plane of benzene therefore, the attack on ortho position by amide nucleophile is not sterically preferred.
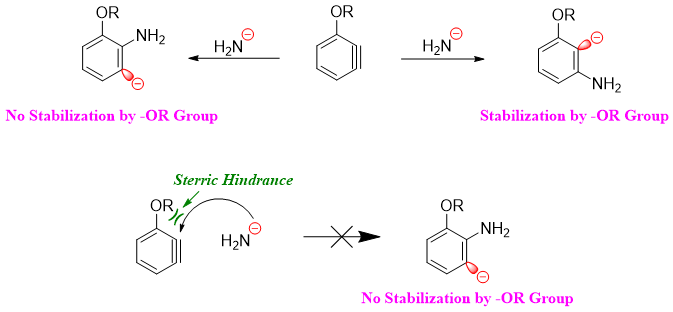
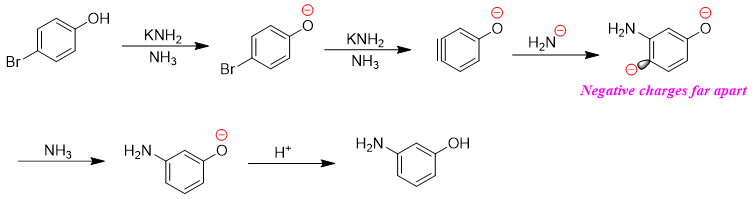
If the substituent is anion and can repel electrons, then the product will be meta substituted. This is since the carbanion at para position is far apart in distance from the negatively charged substituent.
It is possible to synthesize benzyne in the absence of nucleophile so that it is not captured. Following reaction shows the synthesis of benzyne exclusively.
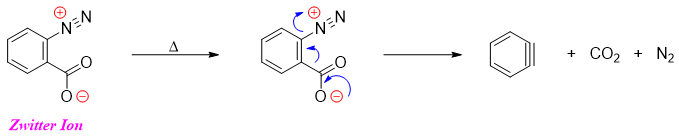
It is not possible to isolate benzyne because it reacts with itself to produce dimer. However, if the above zwitter ion is analyzed in Mass spectrometer it gives a peak of benzyne at m/e 76.