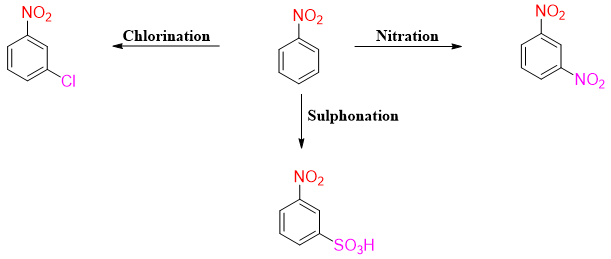
Nitration
Nitration
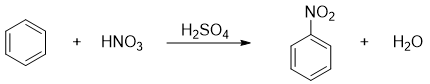
The nitration reaction of benzene was first carried out by German chemist Eilhardt Mitscherlich in 1834. The reaction was employed by reacting benzene with fuming HNO3. Generally, the nitration of benzene is done by reacting benzene with nitric acid in the presence of H2SO4 as a catalyst.
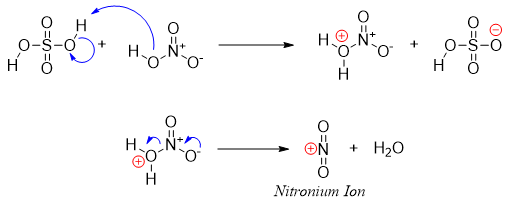
The H2SO4 protonates the nitric acid. The protonated nitric acid loses water molecule resulting in the formation of an electrophile called nitronium ion.
Once generated, the electrophile (nitronium ion) is added to benzene ring via electrophilic aromatic substitution reaction.
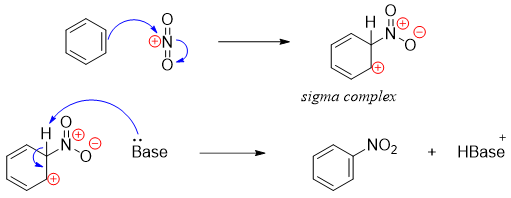
The base present in the reaction mixture can be HSO4-, H2O or solvent. The base deprotonates the carbon to which the nitro group is added (sigma complex) to restore the aromaticity of the benzene ring.
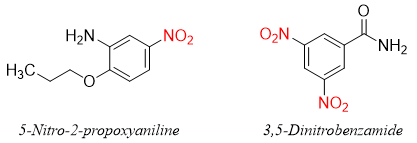
Aromatic compounds containing nitro groups are very important. There are many nitro containing organic compounds used as a medicine and other consumer goods. For example, 5-Nitro-2-propoxyaniline also called as P-4000 or Ultrasüss is used as sweetening agent in food products. Ultrasüss is about 4000 times sweeter than sucrose. Nitromide (3,5-dinitrobenzamide) is a potent antibacterial agent.
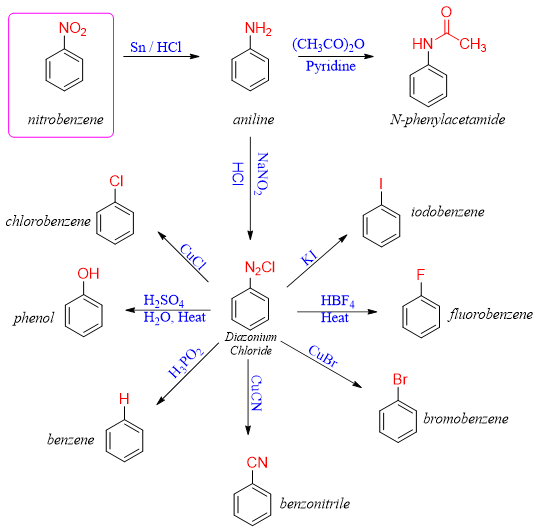
The nitration reaction of aromatic compounds does not occur naturally. This reaction is experimentally performed as the resulting nitro containing compounds are important precursors of different organic reactions. Following scheme shows the conversion of nitro compound into different functional groups.
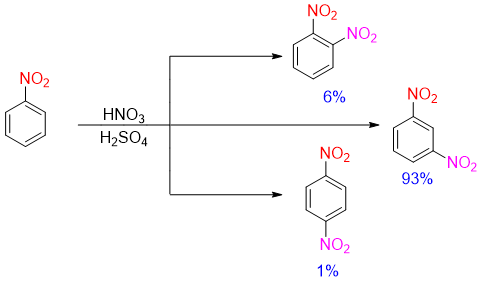
The presence of nitro group on benzene ring acts as a deactivating group. The nitro group is meta directing group. Hence, electrophilic substitution reaction of nitrobenzene will yield 93% 1,3-dinitrobenzene (meta-product), 6% 1,2-dinitrobenzene (ortho-product) and 1% 1,4-dinitrobenzene (para-product).
The nitro group is said to be strongly deactivating. It deactivates the benzene ring towards electrophilic aromatic substitution reaction. The nitro group reduces the electron density on ortho, and para positions as depicted in resonating structures below.
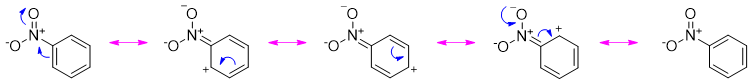
As shown above the positive charges (electron deficiency) are appearing on ortho and para positions hence, leaving the meta position electron rich. Therefore, nitrobenzene undergoes electrophilic aromatic substitution reactions at meta positions.