Transition States
Transition States
For a reaction to occur the reactants must collide in proper orientation and with enough energy to cross the activation energy barrier. Let’s look at the reduction of ketone to an alcohol by reacting ketone with NaBH4.
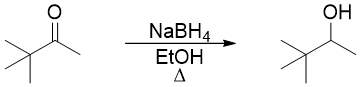
The mechanism of above reaction involves the transfer of hydrogen atom from borohydride to carbonyl group in the first step.
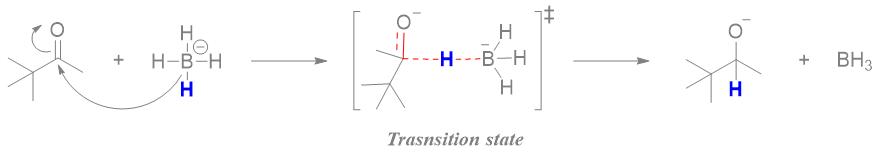
The energy profile for this reaction is shown below.
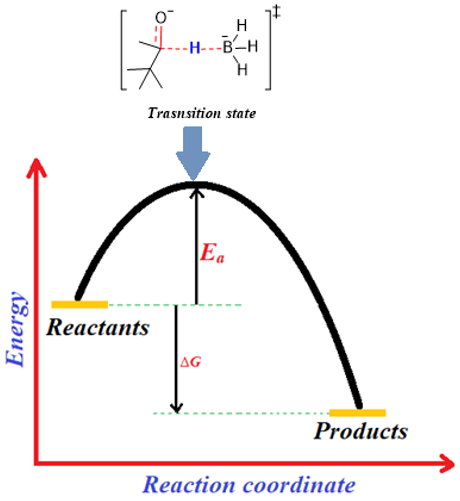
Above energy profile shows that the ΔG of the reaction is negative hence the products formed in this reaction are more stable. To convert into product the reactants have to cross the highest energy point on the profile (activation energy, Ea). This highest energy point corresponds to a specific structure (shown in square brackets) in which the carbonyl bond is partially broken, bond between carbonyl carbon and hydrogen is partially formed, and bond between hydrogen and boron is partially broken. This structure is called as transition state. Transition state is the highest energy point through which the reactants pass to convert into products. The transition state is highly unstable state and cannot be isolated from the reaction mixture.
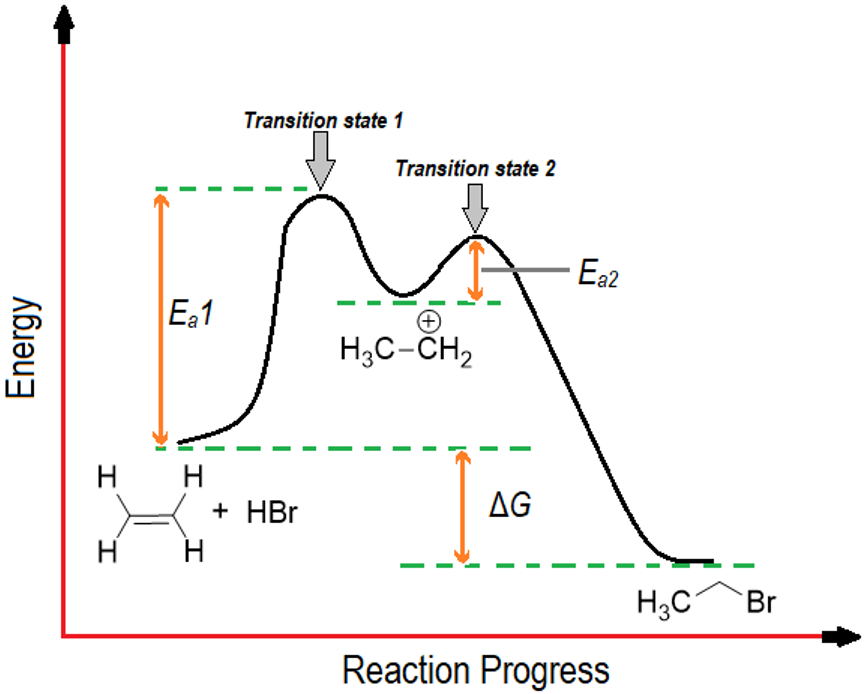
Consider another example involving two transition states. Such reactions have two activation energy barriers. The reactants and products are separated by an intermediate. For example, the addition of hydrogen bromide to bromoethane.
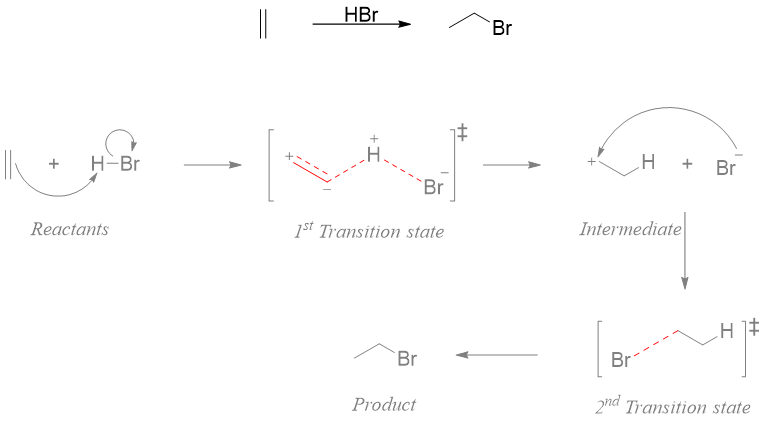
The energy diagram of above reaction shows that in the first step of the reaction the double bond of alkene attacks the electropositive hydrogen of hydrogen bromide. The transition state of this step shows that the bond length between two sp2 hybridized carbon atoms is increasing as it going to change to sp3 hybridized form, the bond between one carbon and hydrogen of HBr is partially forming and the bond between the hydrogen and bromine if partially breaking. First step of the reaction will form a carbocation intermediate. The carbocation is formed for a very short period of time and readily reacts with bromide ion. The second transition state is of less energy as compared to transition state 1 and involves the partial formation of single bond between electropositive carbon atom and bromide ion.