Equilibrium Constant and Free Energy
Equilibrium Constant and Free Energy
In organic chemistry the chemical reactions occur with different rates and extents. In some reactions the reactants are readily converted into products while, some reactions are slow and requires modifications in other factors. In some reactions the reactants completely convert to products while, in some reactions only some of the reactants are converted to products.
Reversible Reactions:
Those reactions in which the reactants are converted to products and products are converted back to reactants simultaneously are termed as reversible reactions. In reversible reactions, equilibrium is attained when the rate of formation of products equals the rate of formation of reactants. At equilibrium, the reaction is said to be completed as no more change in concentration of reactants or products take place. Following examples depict chemical reactions in which the rate of formation of product is equal to the rate of formation of reactants.
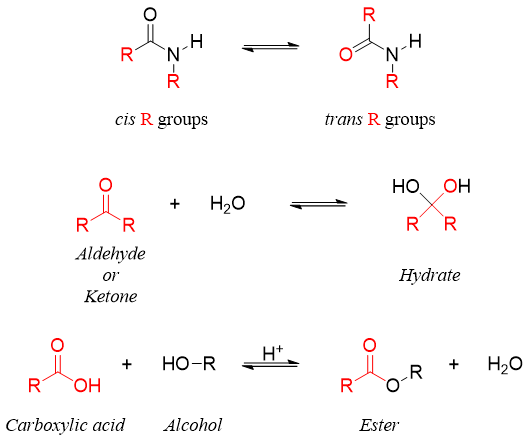
In some reversible reactions the rate of forward reaction is greater than the rate of backward reaction. For example.

While in some reversible reactions the rate of forward reaction is lower than the rate of backward reaction. For example,
Equilibrium Constant:
The concentrations of reactants and products at equilibrium are controlled by the equilibrium constant Keq of the reaction. Consider reversible esterification reaction of carboxylic acids.
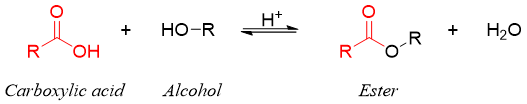
The equilibrium constant Keq for above reaction is defined by equation given below.
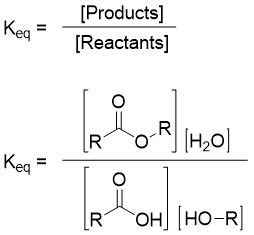
The value of equilibrium constant tells the position of equilibrium, stabilities of reactants and products, and the direction of reaction energetically favored. If the value of equilibrium constant is greater than 1, then the forward reaction is favored (more ester will form). Or if the value of Keq is less than 1, then the backward reaction is favored (more carboxylic acid will form).
In esterification reaction of carboxylic acids, the equilibrium constant value is about 1.
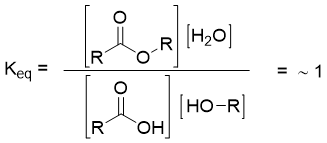
This shows that neither the forward nor the backward reaction is favored.
Whereas the equilibrium constant value for chlorination of methane is 1.1 × 1019.
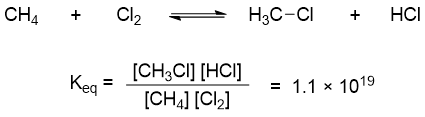
The higher value of equilibrium constant for chlorination of methane suggests that concentrations of reactants at equilibrium are close to zero. Therefore, this reaction is referred to go to completion.
For the esterification of carboxylic acid (Keq = 1) we can manipulate the reaction conditions to synthesize ester with almost 100% yield. According to Le Châtelier's principle, by changing conditions, the dynamic equilibrium of a reaction gets disturbed. To counter the change the position of the equilibrium shifts in either direction. If the concentration of alcohol is increased, the concentrations of ester and water will increase in order to bring down the value of Keq back to 1.
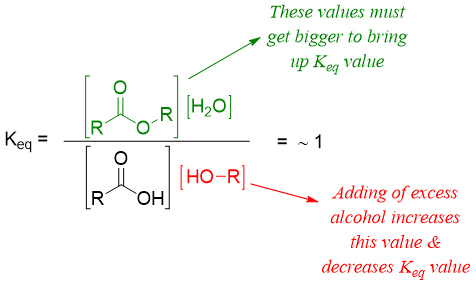
This shows that increasing concentration of alcohol increases the concentration of ester and water. Next, if we remove the water from reaction mixture by different means like using molecular sieves or using Dean–Stark apparatus the concentrations of carboxylic acid and alcohol will go down by producing more ester and water to maintain the Keq value at 1.
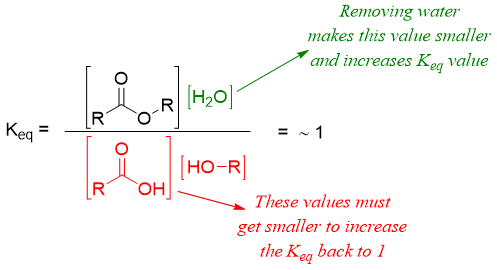
This shows that decreasing the concentration of water will increase the concentration of ester and decreases the concentration of carboxylic acid and alcohol thus moving the reaction in forward direction.
Equilibrium constant and Free energy:
Change in free energy (also called Gibbs free energy) which accompanies a chemical reaction can be calculated from the value of equilibrium constant. Change in free energy is represented as.
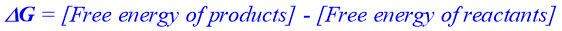
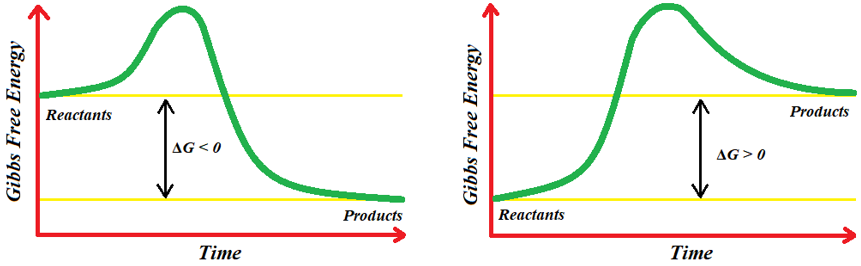
The amount of workable energy is measured by the value of ΔG. If the free energy level of reactants is greater than the free energy level of products, then the ΔG value will be negative and such reactions are spontaneous and proceeds in forward direction. If the ΔG value is zero, then the reaction is at equilibrium. On the other hand, if the free energy level of reactants is smaller than the free energy level of products then the ΔG value is positive and the reaction is non-spontaneous in forward direction but spontaneous in the reverse direction.
When the reactions are carried out using reactants and products in their standard states (pure substance at 25 °C and 1 atm) then ΔG° is commonly used. The relationship between equilibrium constant and ΔG° is given by the expression
Keq = e- ΔG° / RT
Or
ΔG° = -RT(ln Keq)
Or
ΔG° = - 2.303RT (log10 Keq)
According to this equation the Keq increases ( > 1) if the ΔG° value is negative. If the value of ΔG° is positive, then energy must be added to move the reaction in forward direction.
