Kinetics and the Rate Equation
Kinetics and the Rate Equation
In laboratory many reactions are heated to perform a reaction. Heating is rarely performed to alter the equilibrium of a reaction as most of the reactions are not reversible in nature. Reactions are simply heated to speed up them. The study of rates of reactions is called kinetics. Rates of reactions are determined by measuring the decrease in concentrations of reactants or increase in concentrations of products with time.
The rates of reactions usually increase with an increase in temperatures. But the rates of reactions are not only controlled by temperature. If the reacting molecules are not reactive towards each other then the temperature will only increase the rates of collisions and the energy will be lost in the form of heat. Consider following example.
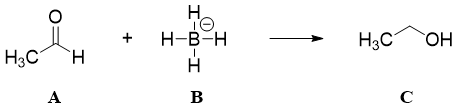
In above reaction there are two types of collisions taking place.
1) Non-productive collisions between A and A.
2) Non-productive collisions between B and B.
3) Productive collisions between A and B.
To increase the collisions between A and B we can simply increase the concentrations of A and B. The greater the concentration of reactants the greater will be the possibility of collisions between reactants and vice versa. The relationship between concentration and rate of reaction is expressed as.
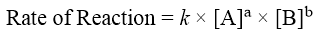
Where k is called as rate constant. Different reactions have different k values, and it changes with change in temperature. The power a and b are called the orders of reactions with respect to A and B. The overall order of reaction is the total of the powers (a + b). The order of a reaction is calculated experimentally. Consider following reaction.
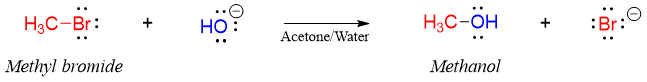
Experimentally when the concentration of methyl bromide was doubled the rate of reaction also doubled. Also, doubling the concentration of hydroxide ion also doubled the rate of reaction. This means that the rate of reaction for above reaction is directly proportional to the concentrations of both methyl bromide and hydroxide.
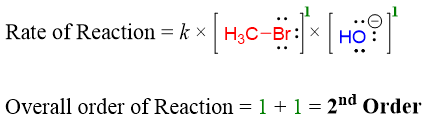
Consider another reaction.

In above reaction when the concentration of tert-butyl bromide was doubled the rate of the reaction also doubled. Whereas, when the concentration of hydroxide ion was doubled there was no effect recorded on the rate of the reaction. Hence, for this reaction the rate equation is written as.
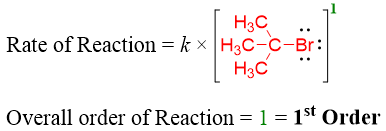
The concentration of hydroxide does not affect the rate of the reaction because the rate determining step (slowest step) only involves tert-butyl bromide. i.e.
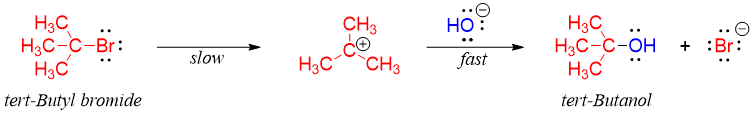
The rate determining step of a reaction has the largest activation energy barrier. The rate determining step is like the turnstiles. It doesn't matter how fast or slowly people leave after passing through turnstiles to empty a railway station; the speed is only limited by the rate at which the turnstiles are operating.
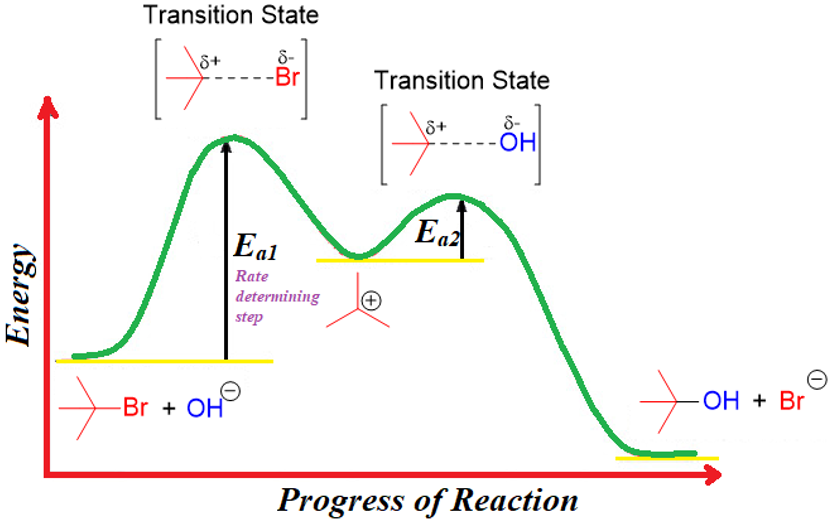
The rate equation is determined experimentally. It cannot be predicted from the stoichiometry of the balanced chemical equation. Once the rate equation is determined then one can propose a plausible mechanism of a reaction.
