The Hammond Postulate
The Hammond Postulate
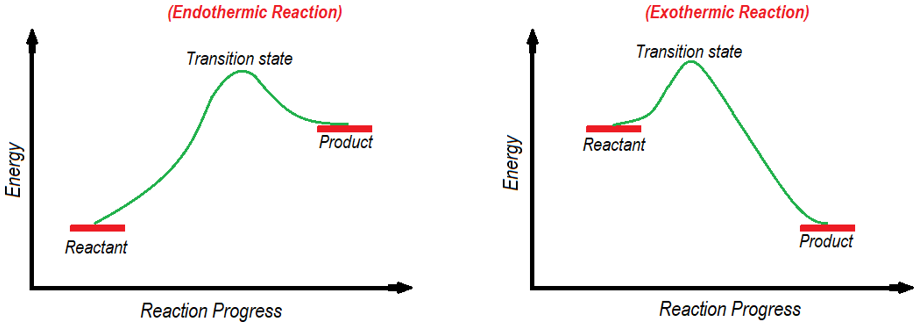
The Hammond postulate provides details about the structure of transition states. According to this postulate the structure of transition state will most likely resemble the reactant, intermediate or product which is close in energy to it. Following energy diagrams shows endothermic and exothermic reactions. In endothermic reaction the transition state energy is close to the energy of the products hence, the structure of transitions state will resemble the structure of product. Whereas, in exothermic reaction the energy of transition state is closer to the energy of reactants hence, the structure of transition state will resemble the structure of reactants.
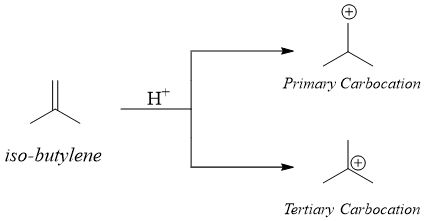
The Hammond postulate can be applied to many organic reactions. In electrophilic addition reactions an electrophile is added to an alkene. For example, when proton is added to iso-butylene it produces two different carbocations. i.e., a primary carbocation and a secondary carbocation.
The energy diagram for the formation of these two intermediates is shown below.
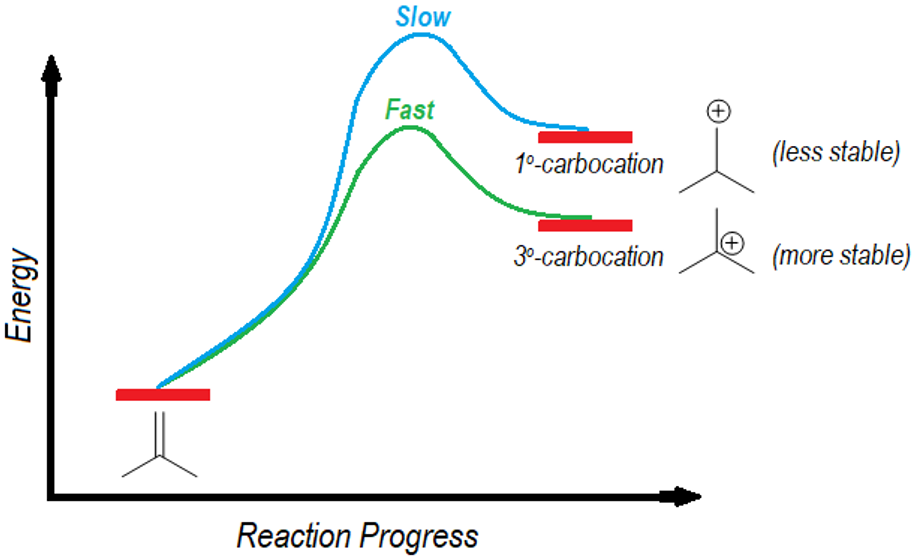
The diagram shows that the protonation of iso-butylene is an endothermic reaction. Hence, the transition states resemble the intermediate carbocations. Thus, the factors that stabilizes the carbocations will also stabilize the transition states. An increase in alkyl substitution on carbocation increases its stability, the same stability is observed in the transition state too. Therefore, the tertiary carbocations are formed faster than the primary carbocations as the transition state energy of tertiary carbocation is less than the transition state energy of primary carbocation.
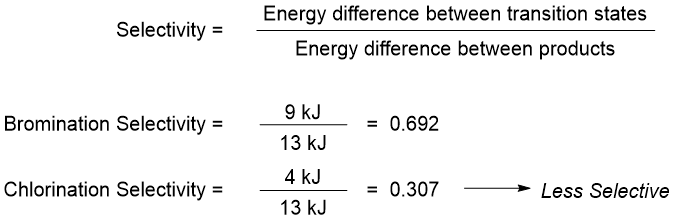
The Hammond postulate also explains the selectivity of bromination over chlorination of alkanes. Following are the energy diagrams for the propagation step of bromination and chlorination reactions.
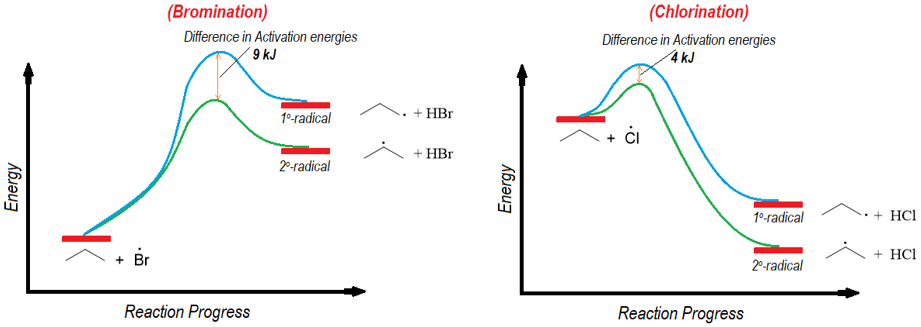
As shown above the propagation step in bromination reaction is endothermic while, in chlorination reaction it is exothermic. Furthermore, the difference between the energies of transition states in bromination is greater than the chlorination reaction. According to Hammond postulate the transition state of bromination reaction will resemble the intermediate structures thus, in transition state the carbon atom will have more radical character. Whereas the transition state of chlorination reaction will experience less radical character.
The energy difference between the two alkyl radicals both in bromination and chlorination reaction is 13 kJ. In bromination reaction the energy difference between two transition state is 9 kJ whereas, in chlorination reaction it is 4 kJ. The smaller transition state energy difference of chlorination shows that both alkyl radicals can form at a given conditions. Hence, the chlorination is less selective than the bromination of alkanes.