Activation Energy
Activation Energy
The surface of the molecule is coated with a layer of electrons present in bonding and non-bonding orbitals. Large molecules repel each other as the surfaces of these molecules are negatively charged. Reactions only take place if the reacting molecules have enough energies to overcome these repulsive forces. That minimum amount of energy required by the reactants to react is called as Activation energy.
The combustion of Petrol (iso-octane) proceeds with ΔG = –1000 kJ/mol at 298 K. The equilibrium constant value for ΔG = –1000 kJ/mol is about 10175. The exceptionally large value of Keq (there are about 1086 atoms in noticeable universe) suggests that in the presence of oxygen the iso-octane simply could not exist.
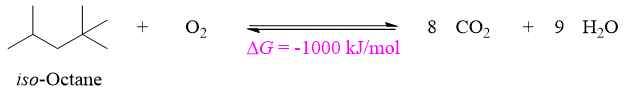
Despite of its highly reactivity towards oxygen we still put petrol in car fuel tanks. The equilibrium is not reached when petrol and oxygen gas are present in the same system. A small amount of energy is required to reach the equilibrium. This energy can be provided by any means including spark by spark plug in car engine, lighting matchstick, spark ignited by a lighter etc. Thus, we can conclude that the mixture of oxygen and petrol is kinetically stable but thermodynamically unstable. This mixture is kinetically stable because despite of having the potential to transform into a more stable, an energy barrier prevents it from doing so. While it is thermodynamically unstable because after combustion reaction the products can never be converted back to iso-octane by providing the same small amount of energy. The small amount of energy provided for the initiation of combustion of petrol is called as activation energy (Ea).
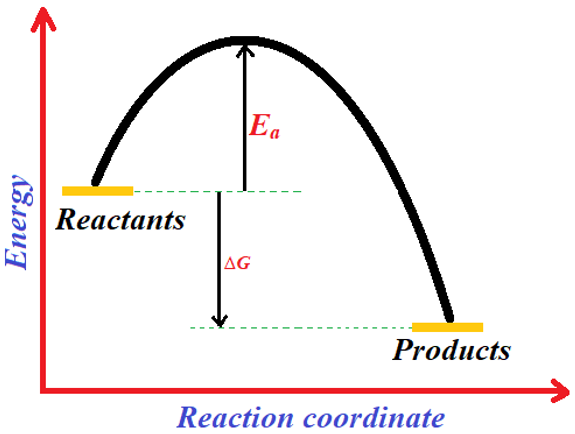
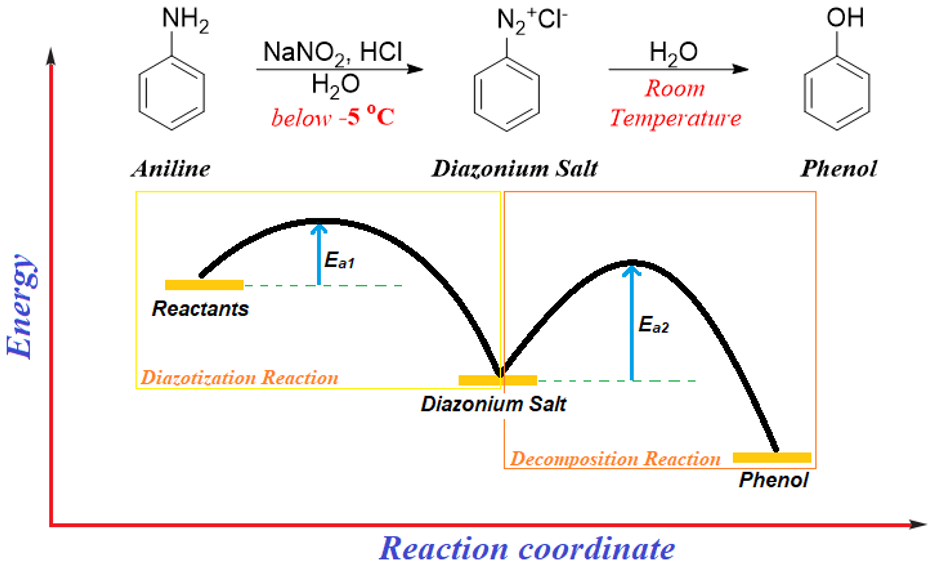
The smaller the activation energy barrier the easier is for the reaction to proceed. If the barrier is large, then energy is provided in the form of temperature to cross the barrier. The rate of reactions always increases with increase in temperature. It is not important to heat every reaction to increase the rate of reaction because this can result in the formation of by-products, or it can result in the decomposition of starting material. Some reactions are even performed at low temperatures. Although lower temperatures decrease the rate of reactions but still, they are used to avoid other side reactions. For example, the diazotization reaction of anilines is performed at low temperatures. In diazotization reaction the diazonium salt forms below -5 °C. This salt is highly unstable and can react with water to form phenol at room temperature. To stop the decomposition of diazonium salt we simply do not provide enough energy so that it cannot cross the activation barrier.
The dependence of rate constant on temperature and activation energy is expressed by Arrhenius equation.
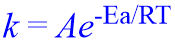
Where;
T = Absolute TemperatureR = Gas constant, 8.314 J.K-1.mol-1
Ea = Activation Energy
A = Frequency factor (constant value)
The frequency factor A explains the number of times reactant collide in proper orientation. Thus, according to Arrhenius equation the rate of reaction depends upon the number of collisions between molecules with proper orientation and their kinetic energy equal to the activation energy. Also, it tells that the rate of reaction increases with an increase in temperature. Following graph explains the relation between temperature and the fraction of molecules having energies to cross the activation energy barrier.
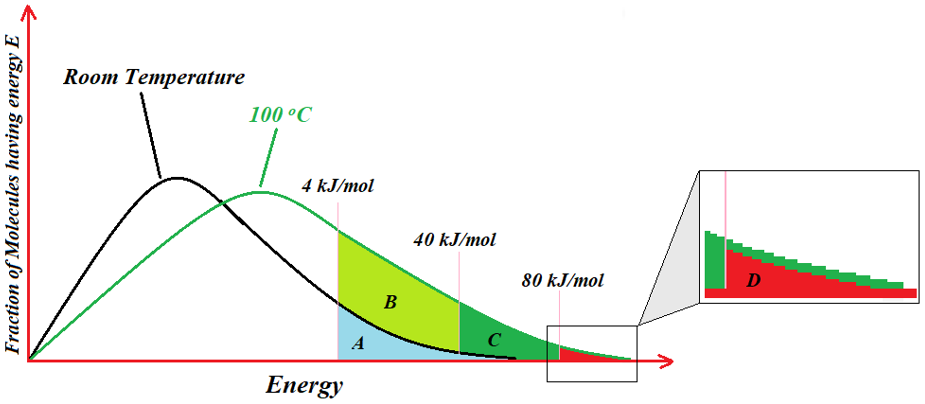
In above graph it can be seen that at room temperature only one fraction (A) of molecules (light blue area under the curve) have energy enough to overcome the energy barrier of 4 kJ/mol. At this temperature none of the molecule can cross energy barriers greater than 4 kJ/mol. When the temperature is increased to 100 °C many of the molecules have the energies enough to cross the energy barriers greater than 4 kJ/mol. For reactions having activation energy barriers ranging between 40 and 60 kJ/mol, a raise of 10 °C in temperature can double the rate of reactions.
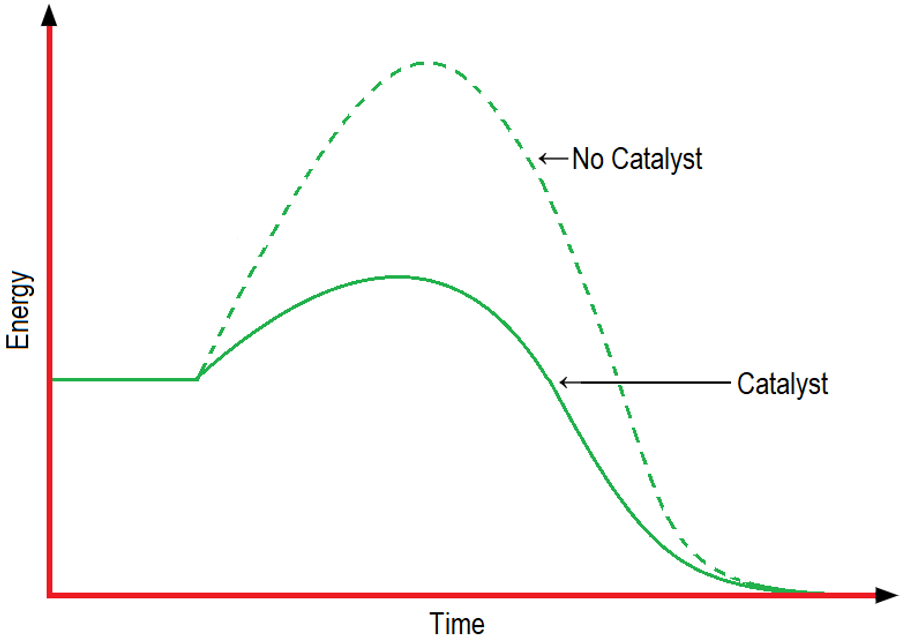
The rate of reactions is also increased by using catalysts. The catalysts decrease the activation energy of a reaction. Catalysts decrease the activation energy of a reaction by different means including decreasing the energy of the transition state or by raising the energy of the starting materials.