Interpreting Energy Diagrams
Interpreting Energy Diagrams
The energy diagrams are used to show the total potential energy of a reaction as it proceeds. On energy diagrams the reactants are present on left hand side and products are present at right hand side.
1) Exothermic and Endothermic Reactions:
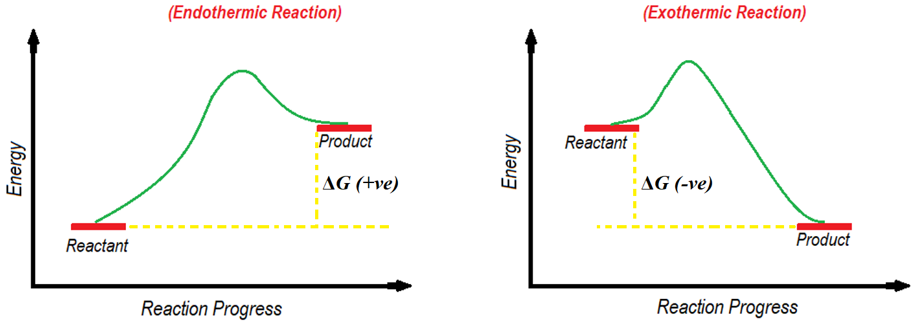
First thing first, find out either the reaction is endothermic or exothermic. To do so measure the change in Gibbs free energy (ΔG) of a reaction. If the ΔG is negative, then the reaction is exothermic or if the ΔG is positive then the reaction is endothermic. If the energy of the product is greater than the energy of the reactant, then the ΔG value is positive. If the energy of product is smaller than the energy of reactant, then the ΔG value is negative.
2) Activation Energy:
Activation energy (Ea) is the minimum energy required by the reactants to start the chemical reaction. On energy diagram the activation energy is the difference between the energy of starting material and energy of the transition state. Transition state is present at the highest point of the curve between reactants and products or reactants and intermediates. Following figure shows the activation energies of endothermic and exothermic reactions.
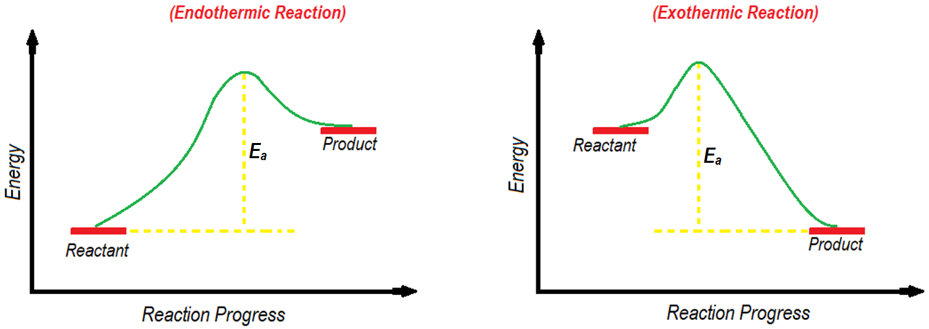
The activation energy is closely related to the rate of reactions. The smaller the activation energy, the faster the chemical reaction will be and vice versa.
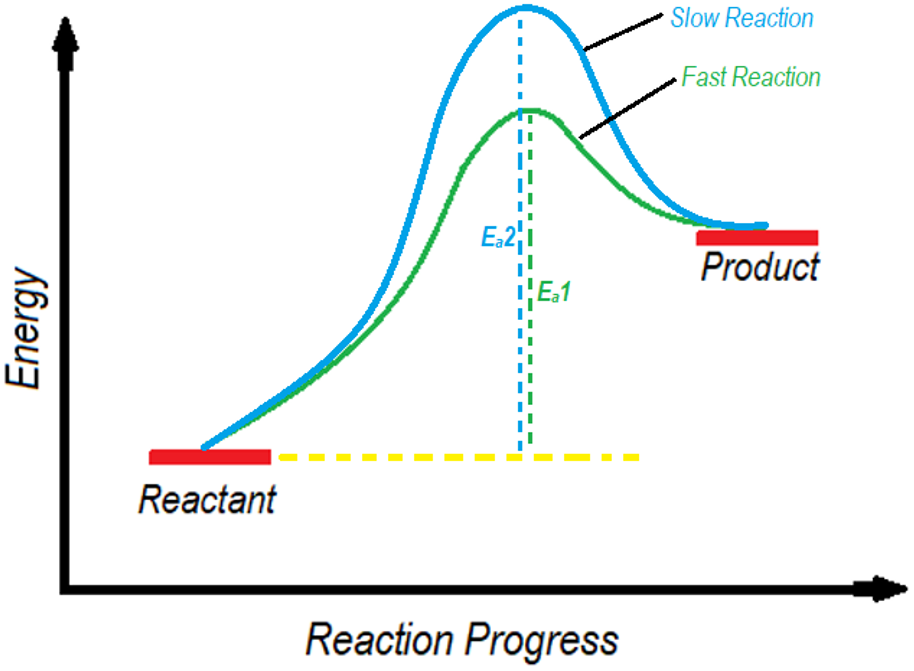
In above diagram the green curved line shows the fastest reaction as it has lower activation energy. Above diagram can be interpreted as the same reaction carried out with and without a catalyst. In the presence of catalyst, the activation energy decreases thus making the reaction to occur fast. While, in the absence of catalyst the activation energy remains higher thus, making the reaction slow.
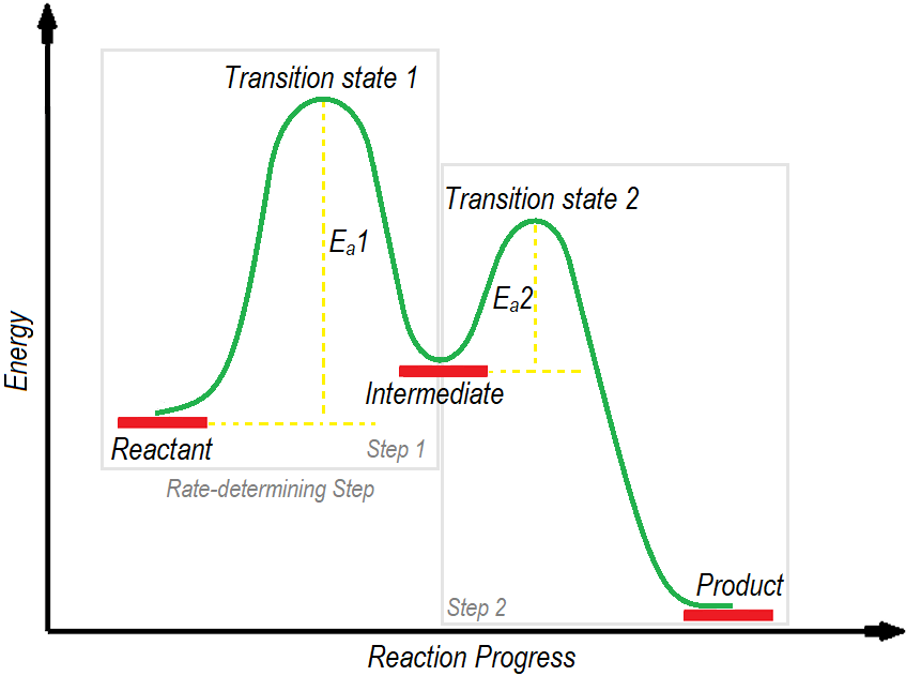
3) Intermediates and Rate-determining step:
Energy diagrams of multiple step reactions are more complex ones. The multiple step reactions involve the formation of intermediates. Intermediates are unstable and highly reactive molecules formed during the reaction for a very short period of time. Intermediates can be in the form of cation, anion, radical, carbene or nitrene etc. Intermediates are stable as compared to transition states and are drawn between two transition states. In a two-step reaction, the energy of the transition state present between reactant and intermediate in first step is greater than the energy of the transition state present between intermediate and the product in the second step. The step of the reaction in which the transition state has the highest energy is termed as rate determining step as it controls the overall rate of the reaction.