Predicting the Outcomes of Acid-Base Reactions
Predicting the Outcomes of Acid-Base Reactions
The best way to predict the equilibrium direction of acid-base reactions for organic molecules is to use pka values. Recall that pKa is just the negative logarithm of acidity constants (Ka).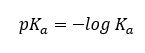
The lower the pKa value of an acid, the stronger the acid, conversely, the higher the pka the weaker the acid. Weak acids and bases are lower in energy compared to strong acids and bases, thus equilibrium direction will move to lower energy weak acids and bases.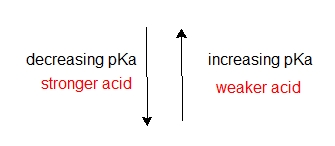
For example, will HCl easily dissociate in water?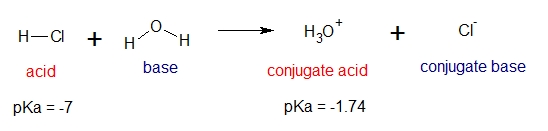
The direction of the acid-base reaction will favor the formation of hydronium ion H3O+ because it’s a weak acid compared to HCl. Since HCl is a strong acid base on its pKa values, then we can say that it will easily dissociate in water.
The following reaction is an equilibrium reaction involving weak acids. Will hydroxide ion react with acetylene?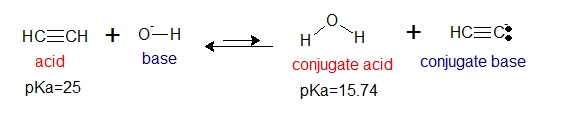
Base on pKa values, we can say that water is a stronger acid compared to acetylene. If we apply what we have mentioned above, the equilibrium will lie to the left. Then we can say that acetylene will not dissociate in water.
