Acid Strength and pka
Acid Strength and pka
Acids differ in their ability to donate H+. The general reaction of an acid (HA) with water is the following:
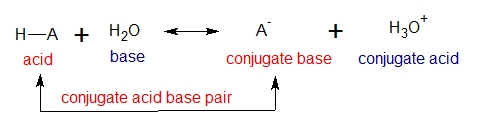
The exact strength of a given acid, HA, in water solution is described using the equilibrium constant Keq for the acid-dissociation equilibrium;
The concentration of water, [H2O], remains nearly constant at 55.5 M at 25OC, thus we can rewrite equilibrium expression using new quantity called the acidity constant Ka. Acidity constant is a measure of acid strength in water. Equilibria for stronger acids favor the products (to the right), thus the stronger the acid, the more it dissociates. Strong acids are almost completely ionized in water, and their dissociation constants are greater than 1.
Since most organic acids are weak acids, the acidity constant is given by the expression Ka. Equilibria for weaker acids favor the reactants (to the left) and thus have smaller acidity constants.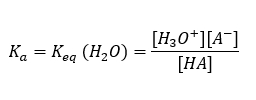
Acid strengths are normally expressed using pKa values. pKa is simply the negative common logarithm of the Ka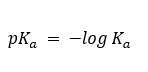
Stronger acids (larger Ka) have smaller pKa around 0 (or even negative), while weaker acids (smaller Ka) have larger pKa values that are greater than 4.
