Lewis Acid and Base
Lewis Acid and Base
The Brønsted–Lowry definition of acids and bases depends on the transfer of a proton from the acid to the base. However, we can look at an acid–base reaction from the viewpoint of the bonds that are formed and broken rather than a proton that is transferred. A general reaction is shown below: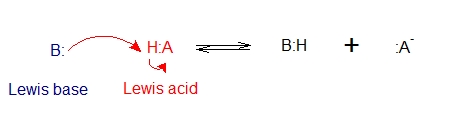
The Lewis definition is broader than the Brønsted-Lowry definition. A Lewis acid is an electrophile, a substance with a vacant low energy orbital that can accept an electron pair from a base. A Lewis base is a nucleophile a substance that donates an electron lone pair to an acid.
Given the reaction below between ammonia and boron trifluoride, electrons in ammonia (NH3) is donated to BF3 thus acting as a lewis base (nucleophile). Since compounds of group 3A elements have unfilled valence orbitals thus they can accept electron pairs and acts a lewis acid (electrophile).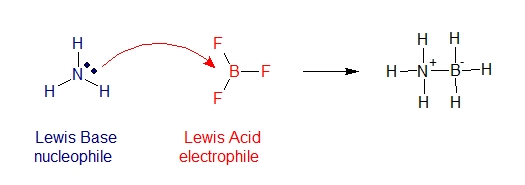
Another reaction presented below, describes why water is the base (nucleophile) as defined by lewis definition and why hydrochloric acid is the acid (electrophile).
Moreover, notice how electrons flow from the electron donor to electron acceptor. It usually described using curved arrows or what is called “curved-arrow formalism”. The curved-arrow formalism is universally used for keeping track of the flow of electrons in reactions. It indicates the direction of electron flow from the base to the acid. Always means that a pair of electrons moves from the atom at the tail of the arrow to the atom at the head of the arrow.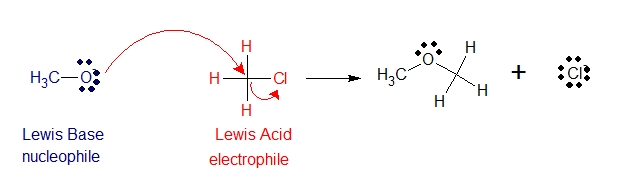
In the reaction above, one curved arrow shows the lone pair on oxygen forming a bond to carbon. Another curved arrow shows that the bonding pair detaches from carbon and becomes a lone pair on the Cl- product.
Aside from a vacant low energy orbital, most Lewis acids have a polar bond to hydrogen so that it can donate H+. Various metal cations, such as Mg2+, are Lewis acids because they accept a pair of electrons when they form a bond to a base. Many transition metals, such as TiCl4, FeCl3, ZnCl2, and SnCl4 are Lewis acids. On the other hand, most oxygen-and nitrogen-containing organic compounds are Lewis bases for they have lone pair electrons.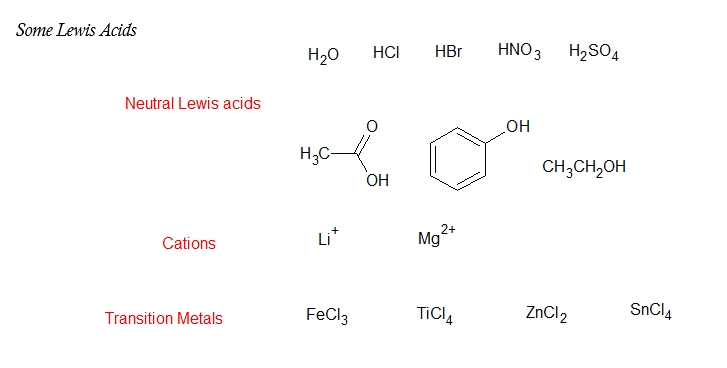
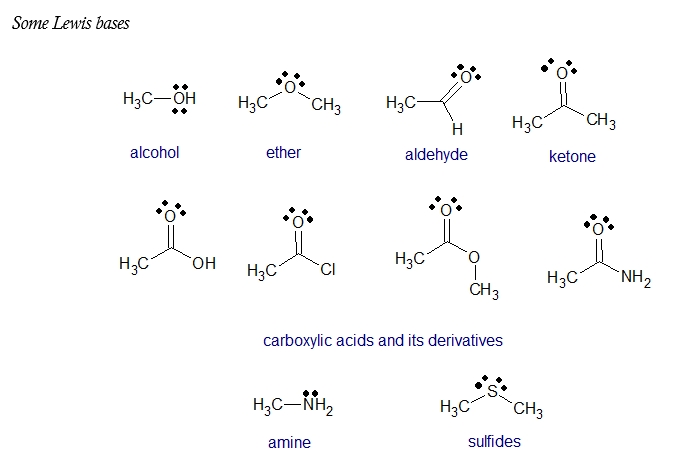
Lastly, it is important to note that the terms acids and bases may have specific meanings in organic chemistry. When organic chemists use the term base, they usually mean a proton acceptor (a Brønsted–Lowry base). Similarly, the term acid usually means a proton donor (a Brønsted–Lowry acid). When the acid–base reaction involves formation of a bond to some other element (especially carbon), organic chemists refer to the electron donor as a nucleophile (Lewis base) and the electron acceptor as an electrophile (Lewis acid).
