Factors to Determine Acid Strength
Factors to Determine Acid Strength
Why do acid strengths of different organic compounds differ? First let’s remember that a strong BrØnsted-Lowry acid loses H+ easily while conjugate base holds on to H+ weakly. Because of this, strong acid have weak conjugate base. A Weak BrØnsted-Lowry acid is one that loses H+ with difficulty and its conjugate base holds on to the H+ strongly thus weak acid has strong conjugate base. To understand why? Let’s consider the different factors that affect acid strength.
Electronegativity
A more electronegative element bears a negative charge more easily, giving a more stable conjugate base and a stronger acid. Electronegativity increases from left to right in the periodic table. Thus the trend would be increasing acidity from left to right. If we compare acidity of methane, ammonia, water and hydrofluoric acid. Fluorine is the most electronegative and Flouride ion is the most stable conjugate base thus hydrofluoric acid is the most acidic.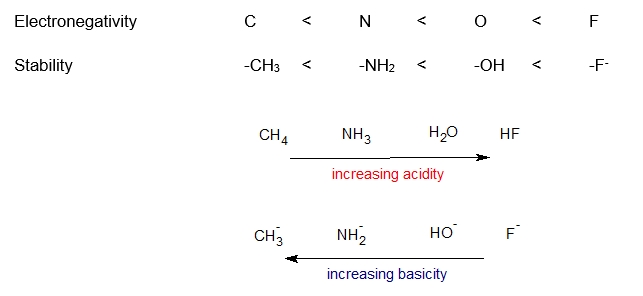
The trend as we go down a group would not quite be the same, for another factor is at play.
Size
The negative charge of an anion is more stable if it is spread over a larger region of space. Within a column of the periodic table, acidity increases down the column, as the size of the element increases.
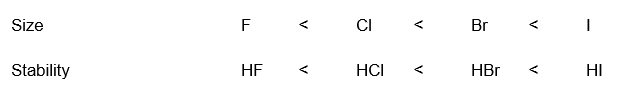
The key is stability of the conjugate base which is also related to another factor which will more stabilized if it’s spread on a larger region of space.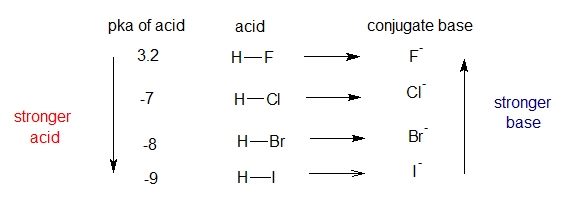
Resonance Stabilization
Now let’s consider how the structure of different organic groups contributes to their relative acidity or basicity, even when we are talking about the same element acting as the proton donor/acceptor.
Let’s consider carboxylic acid and alcohol below
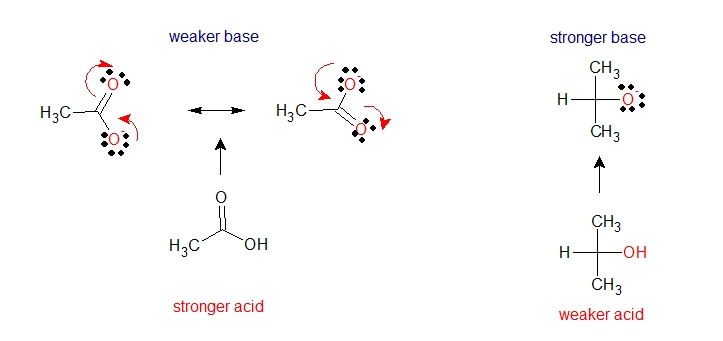
The delocalization of charge by resonance has a very powerful effect on the reactivity of organic molecules. The negative charge of a conjugate base in acetate ion is delocalized over two oxygen atoms by resonance while ethoxide ion has a negative charge localized on one oxygen atom. Resonance delocalization is often the dominant effect helping to stabilize an anion. Thus given the structures above, acetic acid is a stronger acid than alcohol.
Inductive Effects
Electron-withdrawing atoms and groups can also stabilize a conjugate base through the sigma bonds of the molecule. Comparing the acidity of acetic acid and its mono-, di-, and tri-chlorinated derivatives:
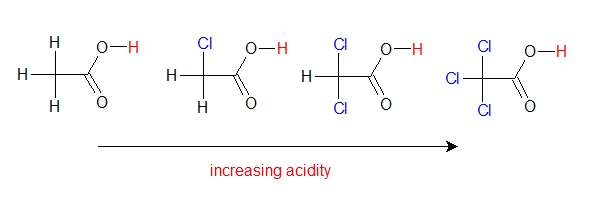
A chlorine atom is more electronegative than a hydrogen, and thus is able to induce or pull electron density towards itself, away from the carboxylate group. The presence of the chlorine atoms clearly increases the acidity of the carboxylic acid group but not because of resonance delocalization, because there’s no more resonance structures for the chlorinated derivative of the acid. Rather, this phenomenon involves something called the inductive effect. In effect, the chlorine atoms are helping to further spread out the electron density of the conjugate base, which as we know has a stabilizing effect.
