Bronsted-Lowry Acids and Bases
Bronsted-Lowry Acids and Bases
The properties and reactions of acids and bases are central to our study of organic chemistry. We need to consider exactly what is meant by the terms acid and base.
Brønsted and Lowry defined acids and bases on the basis of the transfer of protons. A Brønsted–Lowry acid is a substance that can donate a proton [H+], and a Brønsted–Lowry base is a substance that can accept a proton [H+]. Consider the following reaction of hydrochloric acid and water.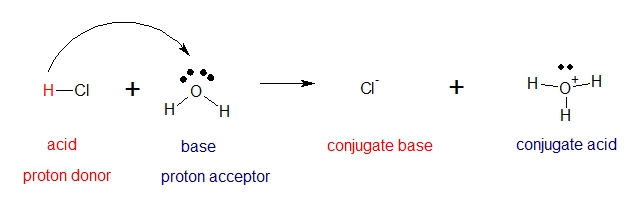
Hydrochloric acid acts as a Brønsted-Lowry acid because it donates a proton [H+] to water and consequently water acts as Brønsted-Lowry base because it accepts the proton [H+] donated by hydrochloric acid. A conjugate acid and a conjugate base is also produced. A conjugate acid is the product that results from protonation of a Brønsted-Lowry base. A conjugate base is the anion that results from deprotonation of a Brønsted-Lowry acid.
In a general sense, you will have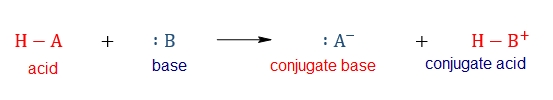
The following are examples of a Bronsted-Lowry Acid-Base Reaction. Another interesting thing to note is that there are substances that can both act as an acid and a base. In the reaction between acetic acid and water, acetic acid donates proton [H+] and acts as a Bronsted Lowry acid and water accepts proton [H+] consequently acting as a Bronsted Lowry base.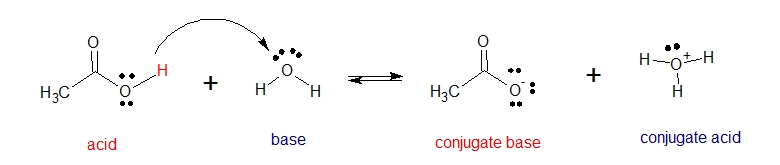
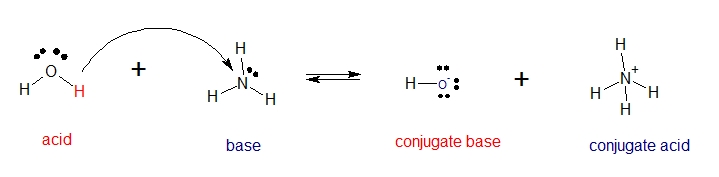
In the reaction between water and ammonia, water donates proton [H+] and ammonia accepts proton [H+]. In this reaction water acts as an acid.