Separation Techniques
Separation Techniques
Enantiomers cannot be separated by using ordinary methods like filtration, distillation, crystallization, normal chromatography etc. as enantiomers have almost the same physical properties. On the other hand, diastereomers have different physical properties therefore they can be separated by general separating methods.
The first ever separation of enantiomers was made by Louis Pasteur. While working on sodium ammonium tartrate crystals he noticed that the crystals were of two different symmetries and shapes. He very carefully separated the two types of crystals by using a pair of tweezers and characterized each sample in polarimeter and found that one solution rotated the plane polarized light clockwise while, the other solution rotated plane polarized light counterclockwise.
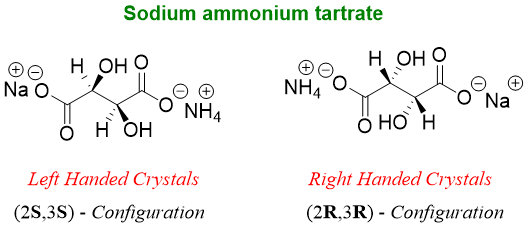
This separation of two enantiomers was termed as resolution of racemic mixture. The name racemic came from racemic acid. Tartaric acid is found in grapes (Latin; racemus = bunch of grapes) thus called as racemic acid.
The racemic mixture is separated into pure enantiomers by following methods.
- Formation of Diastereomeric salts
- Formation of Diastereomeric
- Enzymatic Resolution
- Chiral Chromatography
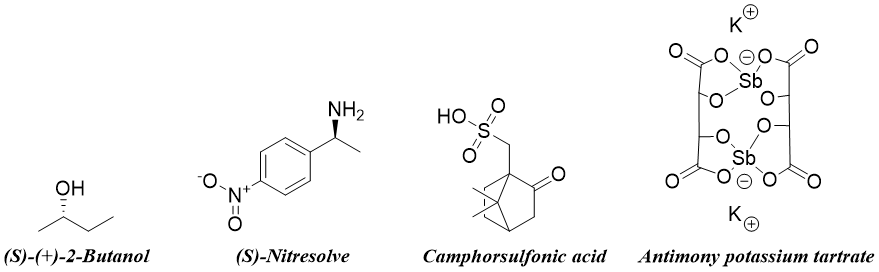
Formation of Diastereomeric salts:
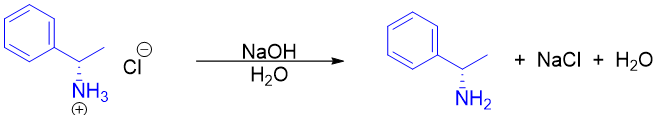
In this method the racemic mixture of a compound is reacted with enantiopure resolving agent to form diastereomeric salts which can then be separated by crystallization. The diastereomers formed have different physical properties. Once both diastereomers are separated they are treated with strong acids to regenerate the pure enantiomer and the resolving agent. For example, the racemic mixture of lactic acid is reacted with (S)-1-Phenylethylamine. The two diastereomers formed are then separated by fractional crystallization and then treated with HCl to regenerate pure enantiomer of lactic acid and (S)-1-Phenylethylamine. After that solvent extraction is performed to separate the two chiral molecules as lactic acid is soluble in organic solvent and ammonium salt of (S)-1-Phenylethylamine is soluble in aqueous layer.
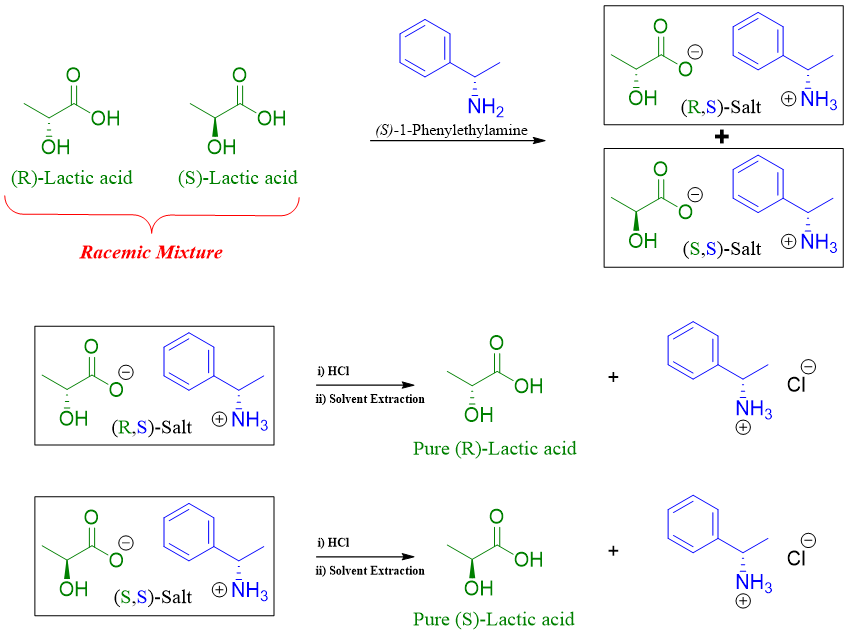
As the enantiopure resolving agents are expensive therefore they are regenerated chemically.
Some other resolving agents are shown below.
Formation of Diastereomeric compounds:
This method is the same as that of diastereomeric salt formation, except in this method the racemic mixture is reacted covalently with enantiopure resolving agents to produce diastereomeric compounds. The diastereomers formed in method are separated by gas or liquid chromatography. One example of this method is shown below.
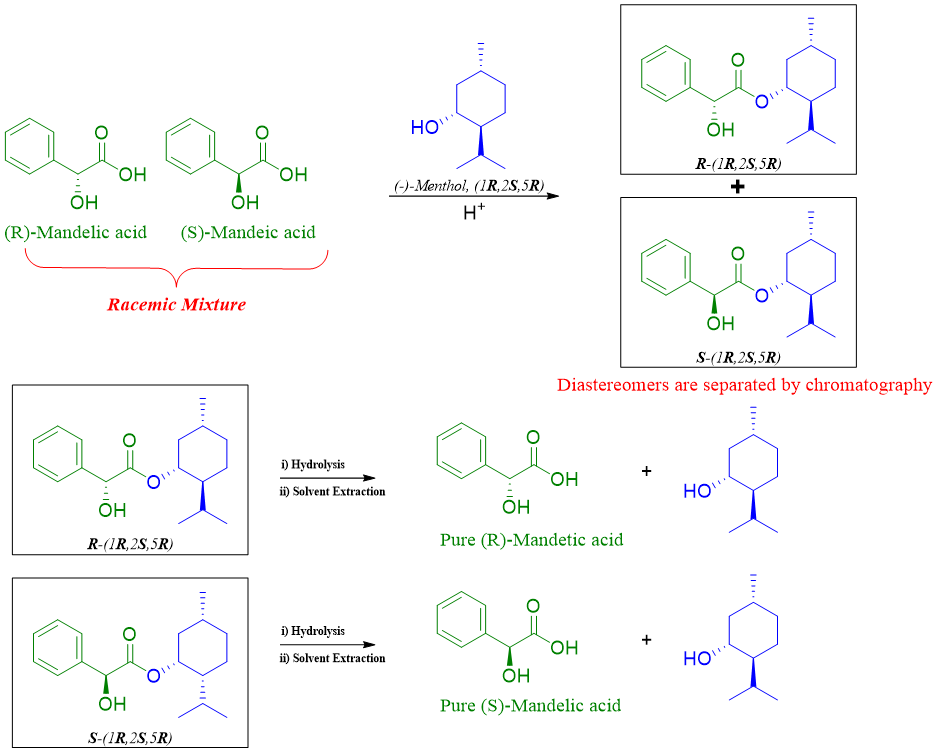
The diastereomeric compounds once separated are then hydrolyzed to produce pure enantiomers and resolving agents.
Enzymatic resolution of racemic mixture:
In this method the enantiomers are separated by reacting them with another compound in the presence of enzymes. The enzyme favorably allows only one enantiomer to react and produce enantioenriched products that can be separated by different conventional separating techniques.
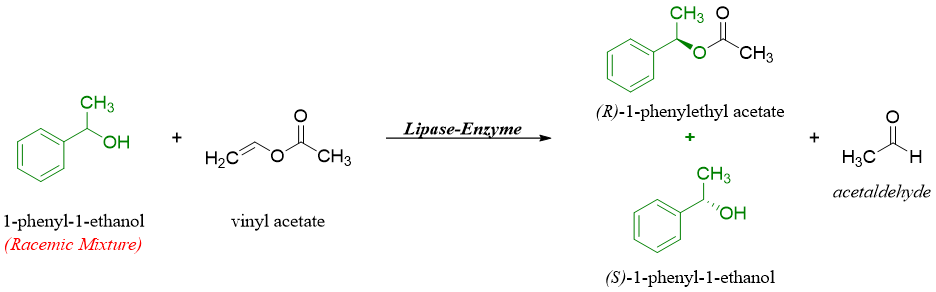
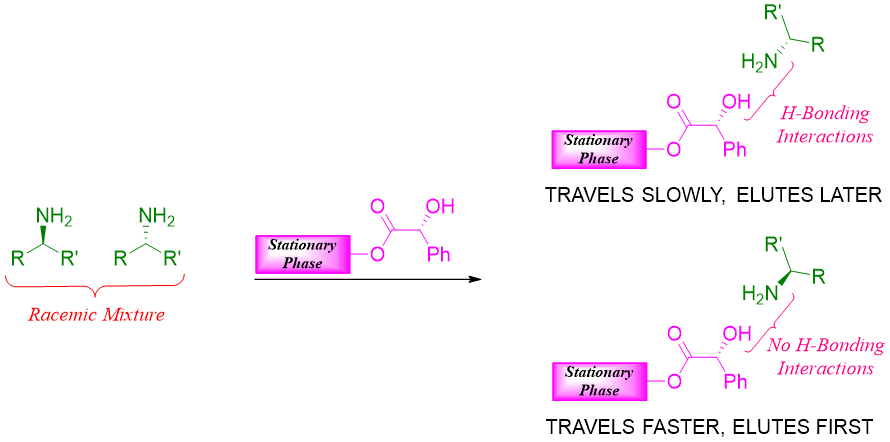
Chiral chromatography:
A more useful method for the resolution of racemic mixture is the use of chiral column chromatography technique. The stationary phase used in liquid or gas column chromatography is chiral. The enantiomers present in racemic mixtures interacts with the chiral stationary phase differently. The enantiomer experiencing more interactions with the chiral stationary phase will travel slowly through the column and will elute out later.