Identifying Stereocenters
Identifying Stereocenters
Stereocenters also called chirality centers or asymmetric centers are sp3 hybridized tetrahedral carbon centers which are bonded to four different types of atoms or group of atoms. The compounds containing chiral centers are optically active i.e., they can rotate the plane polarized light. Following are some examples for identifying the stereocenters.
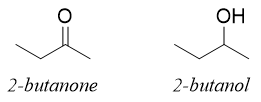
In above two molecules there are four carbons in each molecule. We can write the structure formula of each molecule. i.e.
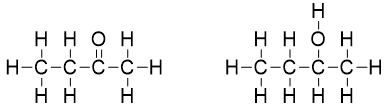
After that we will check chirality (with four different bonded groups) for each carbon atom in both molecules. The carbon atoms highlighted blue are under observation.
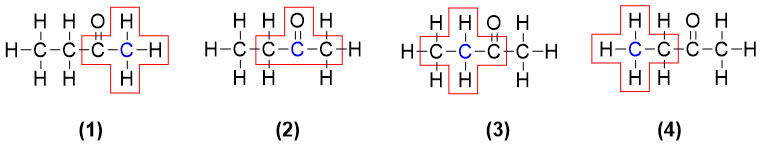
In 2-butanone none of the carbon atom is chiral. As shown above, the C1 (1) is attached to three hydrogen atoms and one carbon atom. As the three hydrogen atoms are identical therefore, C1 is not chiral. The carbonyl carbon C2 (2) is also not chiral as it is sp2 hybridized and is not bonded to four atoms. The C3 (3) is also not chiral because it is bonded to two hydrogen atoms (identical). And the last carbon C4 (4) is also not chiral as it is bonded to three hydrogen atoms. Thus, it is concluded that the molecule 2-Butanone is optically inactive and does not contain any stereocenter.
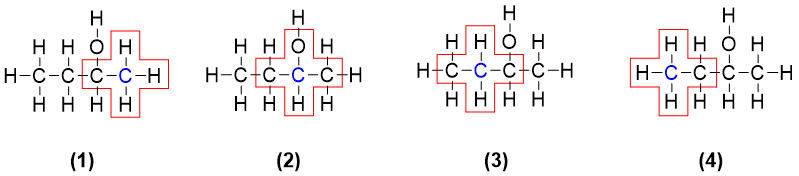
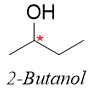
In 2-Butanol there are four carbon atoms. The carbon C1 (1) is not chiral as it is bonded to three identical hydrogen atoms. The carbon C2 (2) is bonded to four different groups i.e., a) H-atom, b) hydroxyl group -OH, c) methyl group -CH3 and d) methylene group -CH2-. Hence, the C2 carbon is a stereocenter and it is optically active. The carbon C3 (3) is not a stereocenter as it is bonded to two identical hydrogen atoms. The carbon C4 (4) is also not a stereocenter as it is bonded to three identical hydrogen atoms. As carbon 2 is a chiral carbon therefore it can be indicated by a star (*).
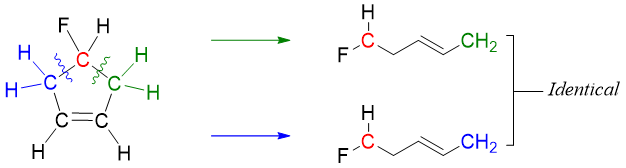
Let’s take another example of 4-fluorocyclo-1-pentene and 3-fluorocyclo-1-pentene.

In 4-fluorocyclo-4-pentene we will exclude the sp2 hybridized carbon atoms as they are not optically active atoms. We are now left with three carbon atoms. Two of the methylene (-CH2)- carbons are not chiral because they are bonded to two identical hydrogen atoms. The carbon to which fluorine atom is bonded is also not a stereocenter because the two alkyl groups attached to this carbon are identical. Hence, making the molecule symmetrical.
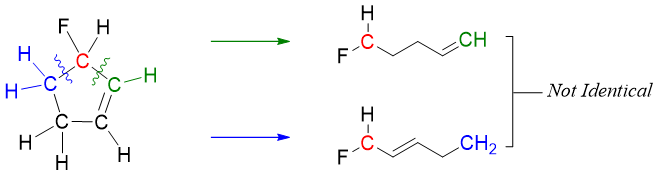
Similarly, in 3-fluorocyclo-4-pentene all of the carbon atoms are optically inactive except carbon bonded to fluorine atom. This carbon is chiral because the molecule is unsymmetrical, and the two alkyl groups are different.
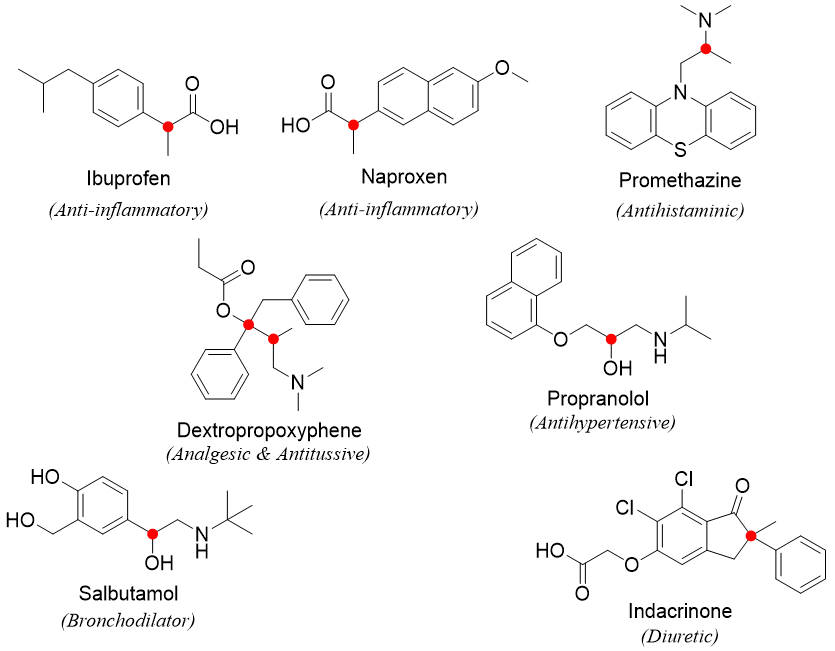
Following are structures of some medicinal drugs containing chiral centers highlighted by a red dot.