Meso Compounds
Meso Compounds
The number of stereoisomers given by compound having n number of chiral centers is mathematically expressed as.
Number of Stereoisomers = 2n
where n = number of chiral carbons
This means that compounds having two chiral centers will have four possible stereoisomers. Each stereoisomer has one enantiomer and two diastereomers as discussed in topic “diastereomers”.
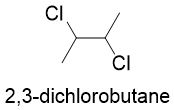
There are some organic compounds that contains two chiral centers but instead of four they only produce three stereoisomers. For example, following four stereoisomers can be drawn for 2,3-dichlorobutane.
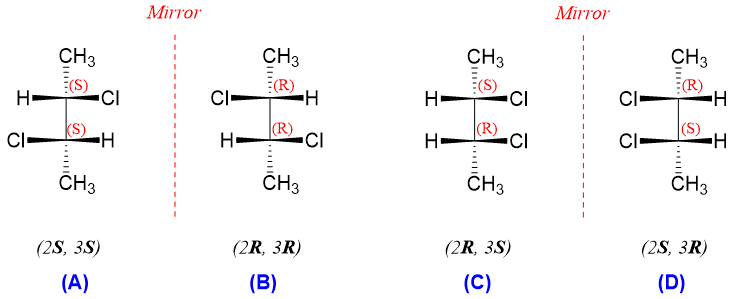
As shown above stereoisomer (A) with 2S and 3S configuration is enantiomer of stereoisomer (B) with 2R and 3R configuration. Meanwhile, the mirror image of stereoisomer (C) with 2R and 3S configuration produces stereoisomer (D) with configuration i.e., 2S and 3R. But experimentally only stereoisomer (C) is found. This is because stereoisomer (D) is the same as stereoisomer (C). This can be explained by following figure.
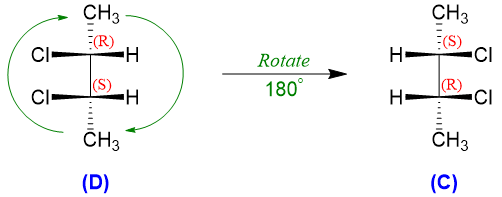
If the stereoisomer (D) is rotated 180 degrees, it will produce the stereoisomer (C). This shows that stereoisomer (C) and (D) are identical molecules and they have a plane of symmetry thus they are referred as achiral despite the fact that it contains two chiral centers. Such compounds which contain chiral centers and are achiral are called as meso compounds. Therefore, 2,3-dichlorobutane exists in three stereoisomeric forms; one meso form and two enantiomers.
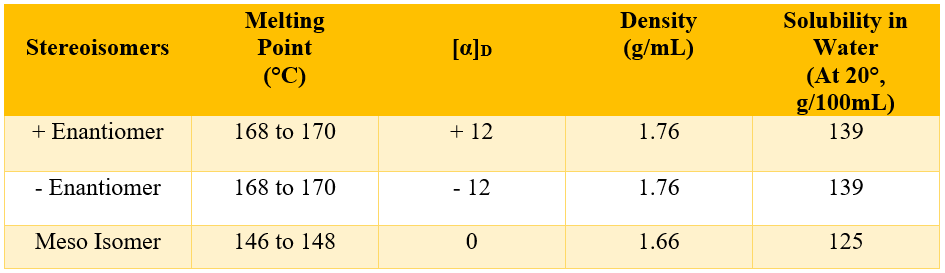
Those compounds which exists in enantiomer and meso forms exhibits different physical properties. For example, tartaric acid also has two enantiomers and one meso form. The enantiomers have almost same physical properties except sign of their rotation of plane polarized light. On the other hand, the meso isomer has totally different physical properties.
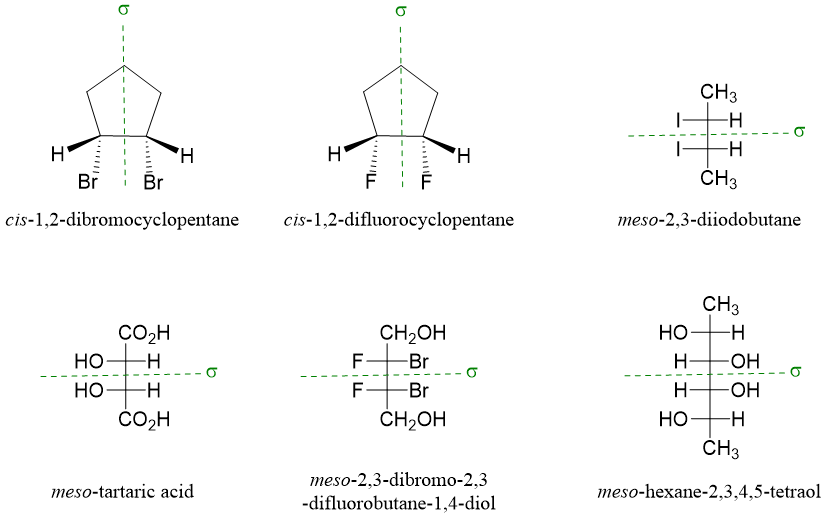
It is easier to identify either a compound containing two chiral centers is a meso compound or not. If there is internal plane of symmetry between two chiral centers, then the compound is achiral and a meso compound. Below given compounds are meso compounds with internal plane of symmetry shown by dashed green line.