Nomenclature of Stereoisomers
Nomenclature of Stereoisomers
Those compounds which have same molecular formula, but different structures are termed as isomers. Isomers are further classified into two main categories: 1) Constitutional Isomers and 2) Stereoisomers.
Constitutional Isomers:
These isomers have same molecular formula but differ in the way their atoms are bonded. For example, 1-propanol and methoxyethane have same molecular formula C3H8O but their atoms connecting pattern is different. In 1-propanol the oxygen atom is connected to hydrogen and carbon atoms while in methoxyethane it is connected to two carbon atoms.
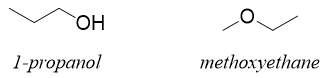
Stereoisomers:
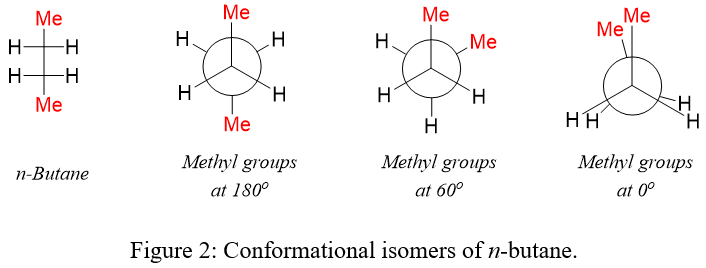
These isomers have same molecular formula, same connecting patterns between atoms but differs in the arrangement of atoms in space. Stereoisomers are further classified as configurational isomers and conformational isomers. In configurational isomerism, the stereoisomers cannot interconvert unless the bond between atoms is broken hence, they can be separated. In conformational isomerism, the stereoisomers quickly interconvert at room temperature (Figure 1) hence, they cannot be separated.
Configurational isomers are of two kinds, 1) cis-trans isomers and 2) isomers with asymmetrical centers.
1) cis-trans (geometrical) isomers:
The cis and trans isomers results due to restricted rotations of bonds. The restriction of rotation can be caused by double bond or cyclic structures. As shown above, in n-butane the bond between C2 and C3 can rotate to produce different spatial arrangements of atoms. In cyclic compounds there is no rotation observed. For example, in disubstituted cyclohexane, the substituents can be present on same side of the ring (cis isomer) or on different side of the ring (trans isomer).
Alkene can also give cis and trans isomerism. The presence of double bond between two carbon atoms restricts it from rotation. For a cis trans isomers of alkene, each sp2 carbon should have different substituents. If any of the two sp2 carbon contain same substituent, then it cannot give cis trans isomers.
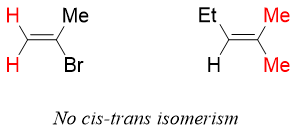
If none of the sp2 carbon is containing same substituents, then it can show cis trans isomerism. If the two hydrogen atoms are present on same side of the plane, then the isomer is cis isomer. If the hydrogen atoms are present on opposite sides of the plane, then it is trans isomers.
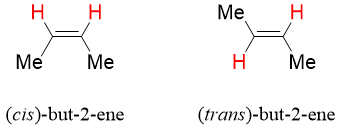
E and Z System:
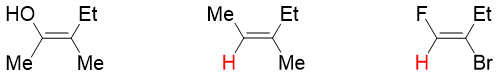
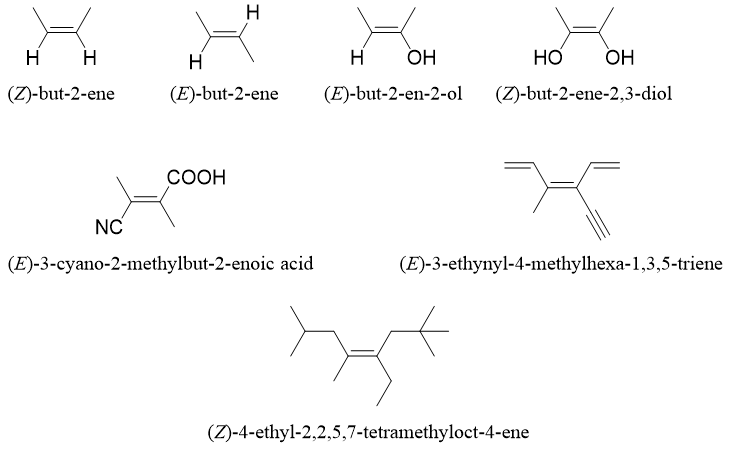
The E and Z system nomenclature is employed for naming alkenes that do not have hydrogen atoms on both sp2 hybridized carbon atoms. For example, following alkene derivatives either contain single or no hydrogen atoms. Hence, they cannot be named by using cis and trans system of nomenclature.
In E and Z system of nomenclature we first calculate the relative priorities of all four substituents attached to sp2 hybridized carbon atoms. In Z isomer, two high priority groups are present (on each sp2 carbon) on the same side of the double bond. Whereas, in E isomer, the two high priority groups are present on opposite sides of the double bond.

Following rules explain determining relative priorities.
a) The greater the atomic number of atoms directly attached to sp2 carbon, the higher is the priority and vice versa. For example, oxygen has atomic number of 8, nitrogen has atomic number 7, carbon has atomic number 6 and hydrogen has atomic number 1. Therefore, oxygen has the highest and hydrogen has the lowest priority.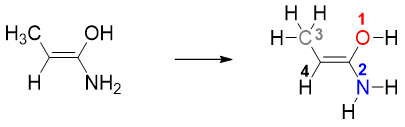
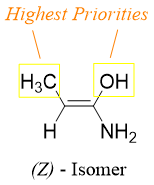
Once the priorities are determined, find the groups with highest priority on each sp2 carbon. If the two highest priority groups are on same side, then the isomer is Z or if they are on opposite side then the isomer is E.
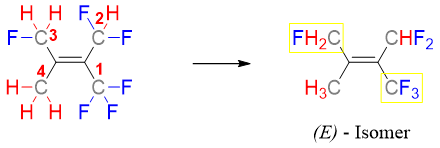
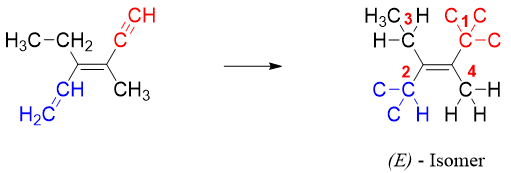
Following are examples of geometrical isomers along with nomenclature.
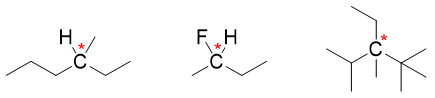
2) Isomers with asymmetrical centers:
Asymmetrical centers are also known as chiral center. Chiral carbon is a carbon atom bonded to four different atoms or group of atoms. The chiral carbon is indicated by a star (*). For example,
Organic compound with single chiral center can exist as two stereoisomers. The two stereoisomers are comparable to the right hand and left hand. The two stereoisomers are also called as enantiomers. The two stereoisomers are mirror images of each other. For example,
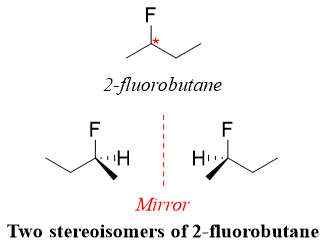
R and S system of Naming Enantiomers:
a) Determine the relative priorities of four groups attached to the chiral carbon. Check out determination of priority in E and Z system above. For example, for naming given stereoisomer of 2-fluorobutane, we will first assign priority order.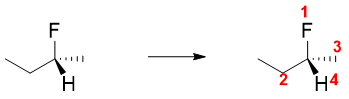
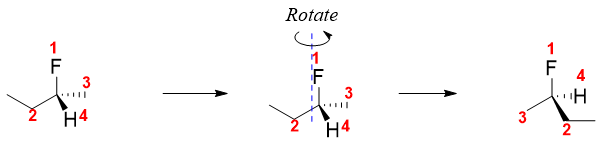
After this draw an arrow starting from priority-1 going through to priority-2 to priority-3. If the rotation is clockwise, then the compound has R configuration or if the rotation is anti-clockwise then the compound has S configuration.
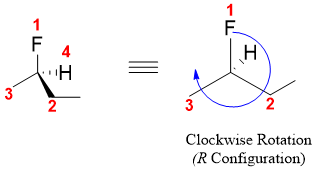
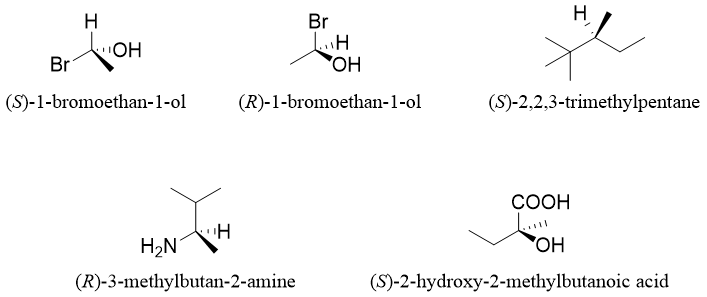
Similarly, the other enantiomer will be having S configuration. Following are some examples of optically active compounds along with their R and S system names.