Synthesis of Epoxides
Synthesis of Epoxides
Epoxides are three membered ring systems in which two vertices are carbon atoms and the third one is oxygen atom. Epoxides are generally synthesized from alkenes, and they undergo variety of valuable synthetic reactions.
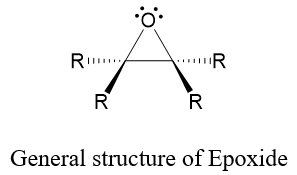
Following are some methods usually employed for the synthesis of epoxides.
1) Epoxidation of Alkene:
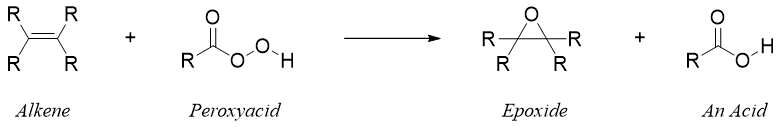
The conversion of an alkene to epoxide is an oxidation reaction. The mild oxidizing agent used for the epoxidation of an alkene is peroxyacid. This reaction is also called as Prilezhaev epoxidation.
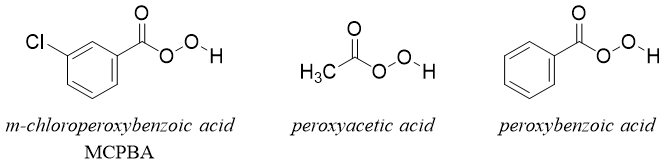
Following are few examples of different peroxyacids.
The reaction of alkene with peroxyacid proceeds without the formation of any intermediate. The epoxidation of alkene takes place in concerted fashion in which the breakdown and formation of bonds take place simultaneously.
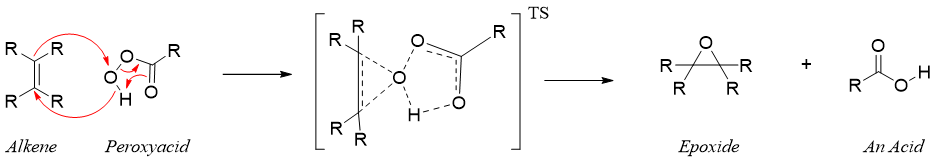
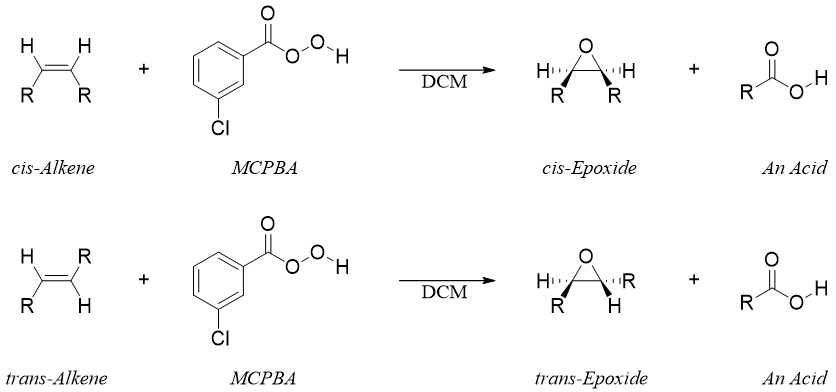
As the epoxidation reaction proceeds without the formation of any intermediate therefore, the stereochemistry of an alkene is preserved. For example.
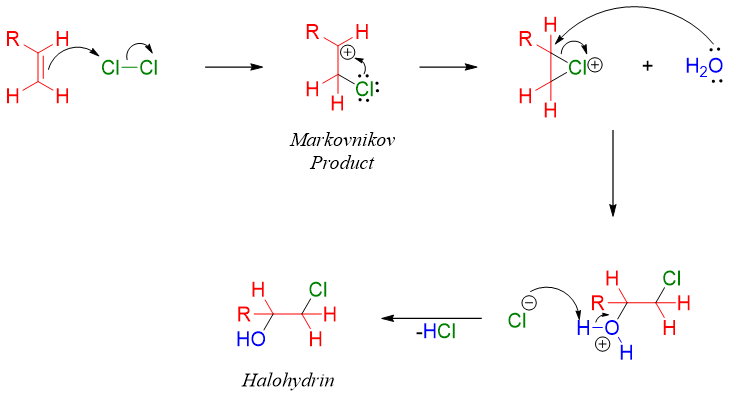
2) Halohydrin to Epoxides:
Epoxides can also be prepared from cyclization of halohydrins. This method is the variation of Williamson ether synthesis. In halohydrins the halogen atom and hydroxide groups are present on adjacent carbons in a molecule.
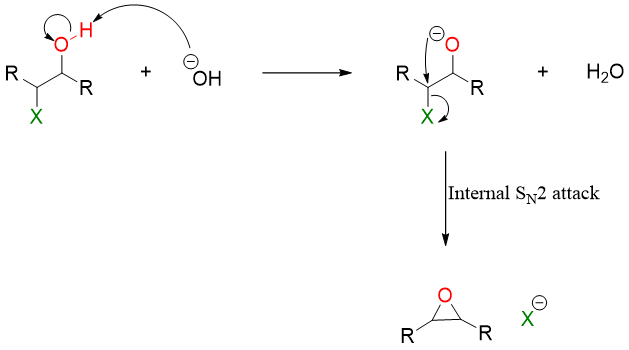
Halohydrin when treated with base produces alkoxide ion. The alkoxide can attack on the carbon to make halogen leave and form epoxide through internal SN2 reaction.
Halohydrins are prepared by reacting alkenes with aqueous solution of halogens.
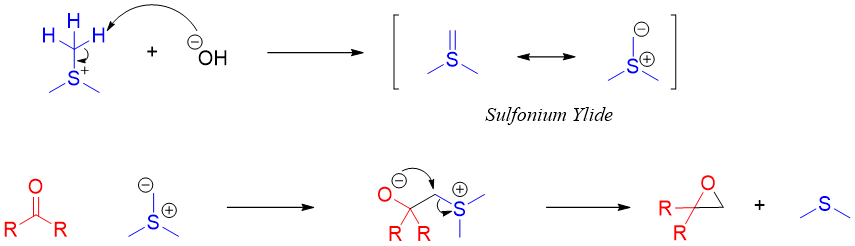
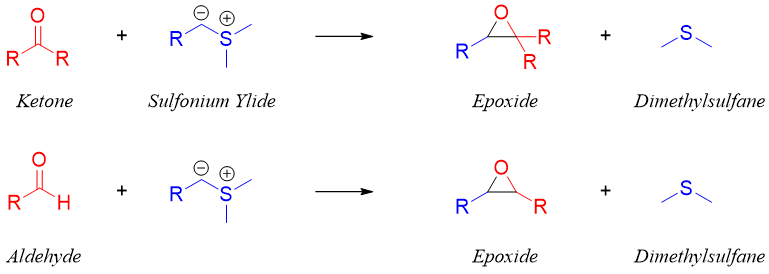
3) Corey–Chaykovsky reaction:
In this reaction aldehydes or ketones are reacted with sulfonium ylide to form an epoxide. This reaction is diastereoselective in nature and favors trans products.
Mechanism: