Sharpless Epoxidation of Alkenes
Sharpless Epoxidation of Alkenes
Sharpless epoxidation is a type of enantioselective reaction employed to prepare epoxides of primary and secondary allylic alcohols. This reaction involves tert-butyl hydroperoxide (t-BuOOH) as the oxidizing agent and a catalyst formed from titanium tetra(isoprpoxide) [Ti(O-i-Pr)4] and diethyl tartrate (DET).
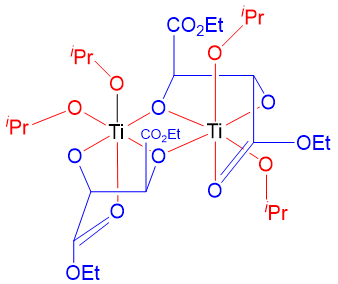
This method can only be used for the enantioselective epoxidation of allylic alcohols. Hence, this is one of the major limitations of this reaction. This reaction works by the formation of an active complex by two titanium atoms bridged by two diethyl tartrate ligands. This reaction works when each titanium atom retains two isopropoxide ligands and make coordinate bonds with carbonyl groups of diethyl tartrate ligands. Following complex is formed by the Ti(O-i-Pr)4 and L-(+)-(DET).
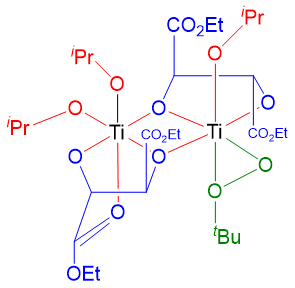
Above dimeric complex is formed when titanium and tartarate are stirred for some time. When oxidizing agent tert-butyl hydroperoxide is added to this mixture it displaces one of the carbonyl groups of tartrate and one isopropoxide ligands.
Once the oxidizing complex is formed the hydroxyl oxygen of allylic alcohol coordinates with the titanium atom by displacing another isopropoxide ligand as shown below.
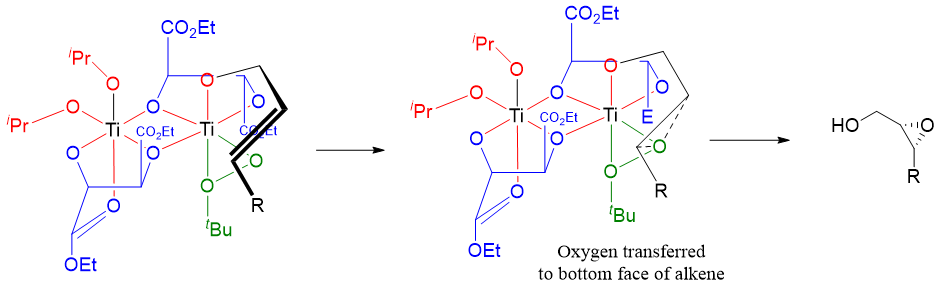
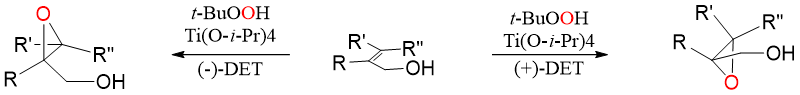
As shown above the reactive oxygen adds to alkene from the bottom face because of the shape of the complex. The epoxide is formed in high enantiomeric excess. The cycle repeats as another hydroperoxide displaces the product. In above example we have used L-(+)-diethyl tartrate which adds the oxygen to the bottom face of the alkene whereas, the D-(-)-diethyl tartrate will deliver oxygen to the top face of alkene. Furthermore, Sharpless found that this reaction only needs catalytical amounts of titanium complex because the product is displaced by more and more reagents and hence, the cycle repeats.
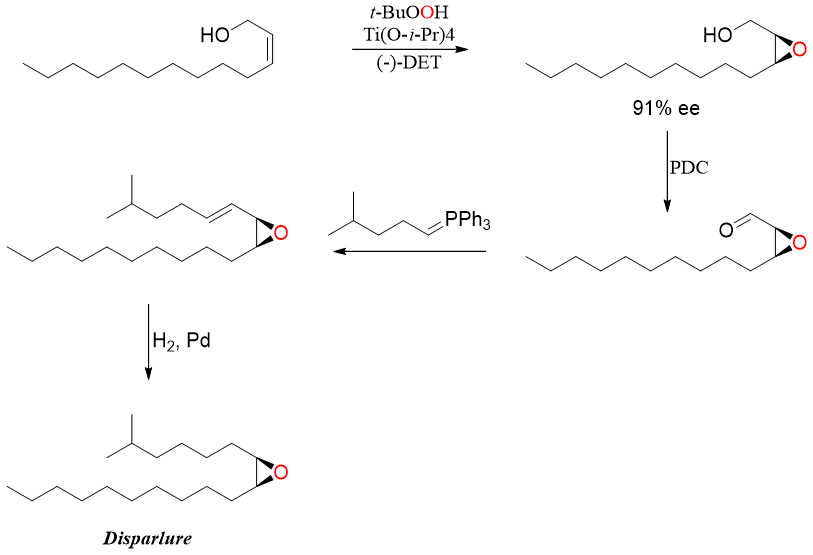
Sharpless epoxidation reaction has many applications. An American company J. T. Baker synthesized synthetic disparlure which is the sex pheromone of gypsy moth. Disparlure is used to detect populations of moths and make them stop from harming plants and forests.
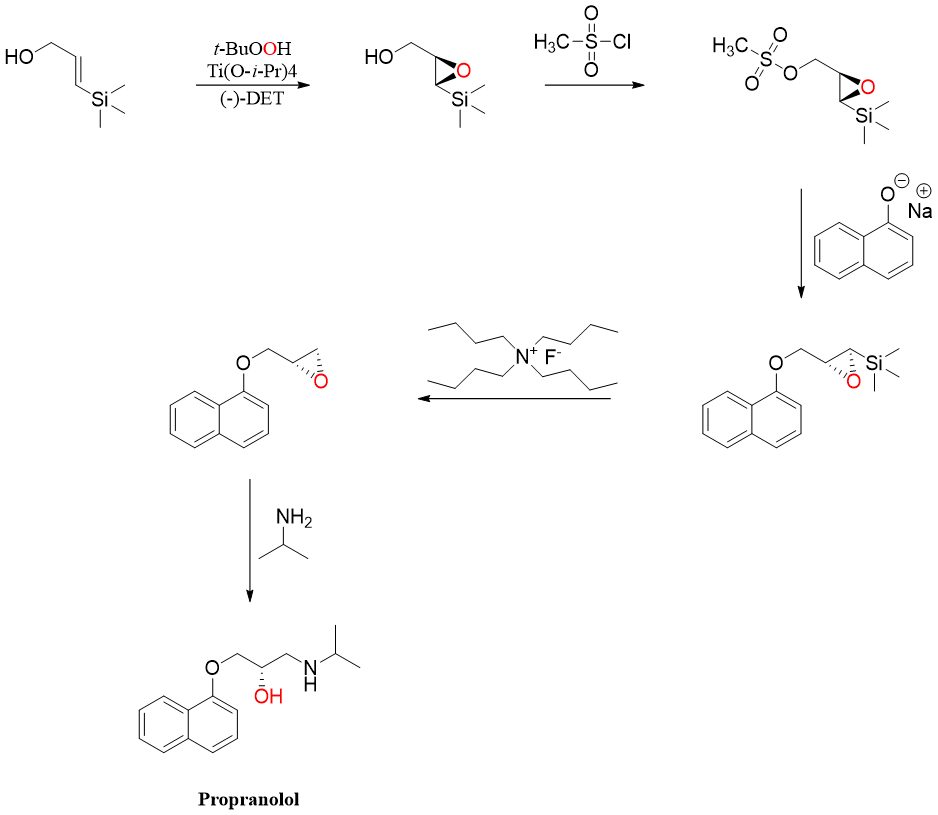
Another problem related to this reaction is the handling of epoxide of allylic alcohol. Sharpless used silicon substituted allylic alcohol to overcome this problem in the synthesis of Propranolol, a chiral beta blocker drug.