Nomenclature of Epoxides
Nomenclature of Epoxides
Epoxides are cyclic organic compounds consisting of three atom ring system. This three atom ring system contains one oxygen atom hence, epoxides are cyclic three atom ring ethers. Due to ring strain, epoxides unlike ethers are highly reactive towards nucleophilic addition reactions.
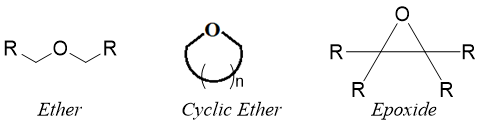
There are two different methods of naming epoxides.
1) Synthesis method:
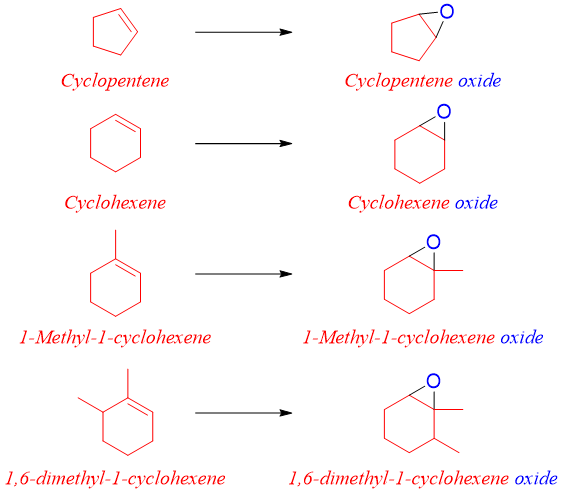
Epoxides are often synthesized from alkenes. Therefore, they are named as oxides of corresponding alkenes. i.e.
The epoxide is named by naming the alkene as the root name followed by addition of suffix oxide. This method is usually employed for naming simple epoxides. For example,
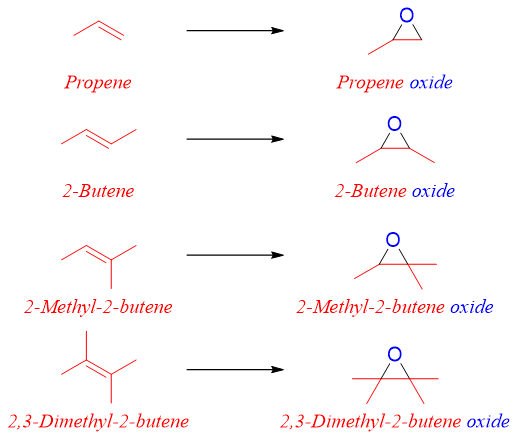
Same method can be employed to name cyclic alkene oxides. For example.
2) Epoxide as Substituent:
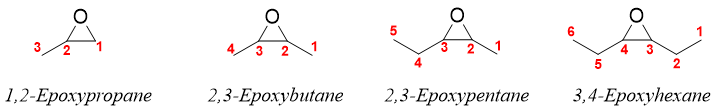
Another method of naming epoxides is taking epoxide as a substituent. The word -epoxy is used to indicate the epoxide functional group. The root name is based on the longest carbon chain containing epoxide functional group. The chain is numbered in such a way that the epoxide functional group gets the lowest possible number. The prefix -epoxide is inserted before the root name along with the carbon numbers to which the oxygen atom is bonded. For example,
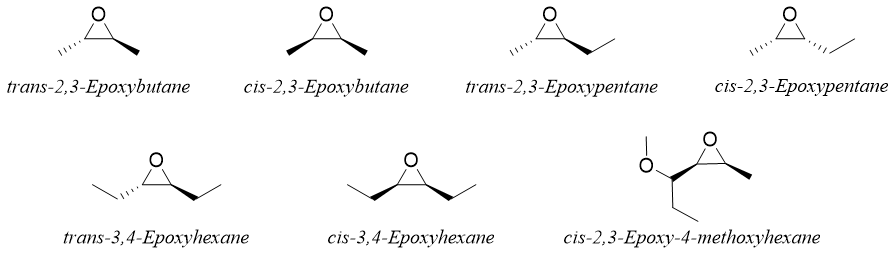
The prefix -cis or -trans is employed to indicate the stereochemistry of epoxide. For example.
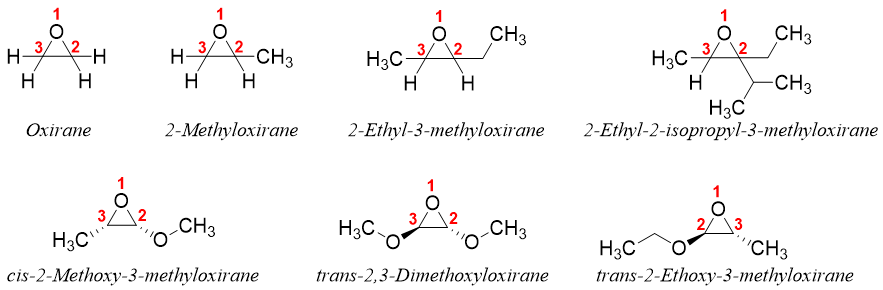
3) Oxirane Method:
Ethylene oxide is also systematically named as oxirane. In this method the numbering of the molecule is started with the heteroatom (oxygen atom) and going in the direction to assign the substituents the lowest possible number. For example.