Epoxide Opening Reactions
Epoxide opening Reactions
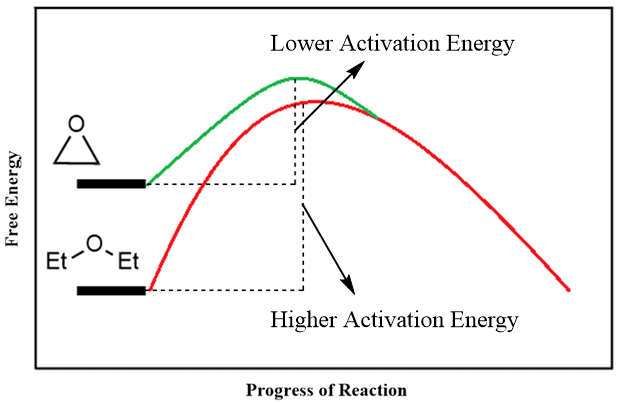
Unlike ethers, epoxides are much reactive towards nucleophilic substitution reactions. This reactivity is due to the ring strain present in three membered ring of epoxide. Upon reaction with nucleophile, the epoxide ring opens hence, relieving the ring strain. Following energy diagram shows the energy profile of reaction of hydroxide ion with ethylene oxide and diethyl ether. The diagram shows that the epoxide requires less activation energy than diethylether.
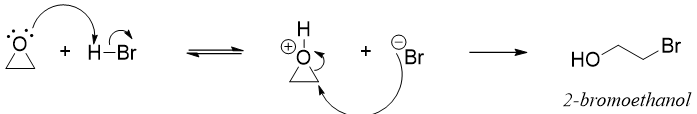
Epoxides react with hydrogen halides to produce halohydrin.
The protonated epoxides are highly reactive and can be opened by any nucleophile including poor nucleophiles like water and alcohols.
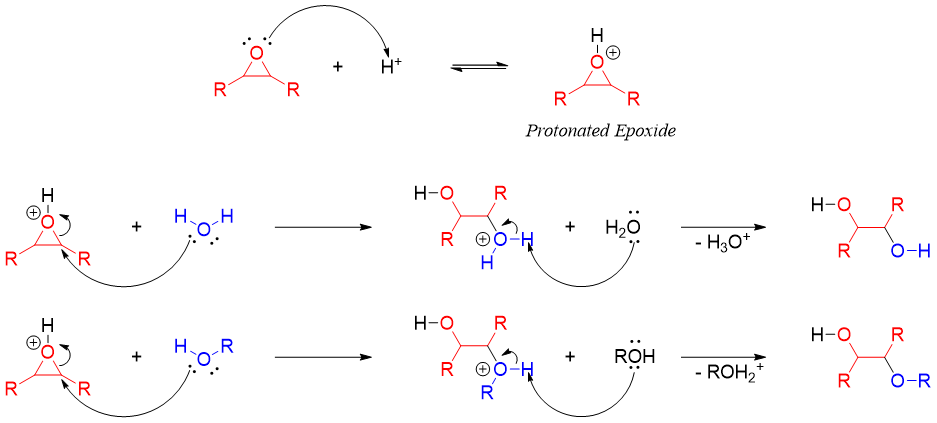
If the epoxide contains two different substituents (unsymmetrical epoxide) and the attacking nucleophile is different from water, then the reaction will produce two different products. The major product is the product resulting from the attack of nucleophile on more substituted carbon. For example.
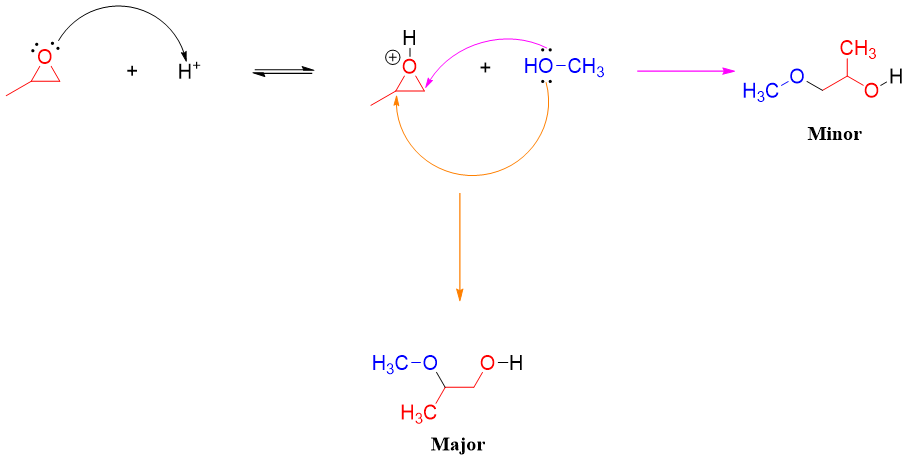
In acidic conditions, the protonated epoxide prefers nucleophilic attack on more substituted carbon atom because the alkyl groups increase the electrophilic character of carbon atom attached to oxygen atom in epoxides. The greater the number of alkyl groups the greater is the stability of resulting carbocation. This makes the C-O bond in epoxide much weaker and hence breaks easily. The C-O bond breaks even before the nucleophile attacks.
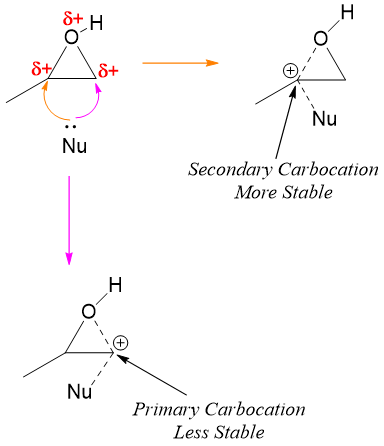
Therefore, it can be concluded that opening of epoxides in acidic medium partially follows both SN1 and SN2 mechanisms.
In basic or neutral conditions, the opening of epoxides purely follows SN2 pathway. Following reaction is an example of opening of epoxide in basic medium.
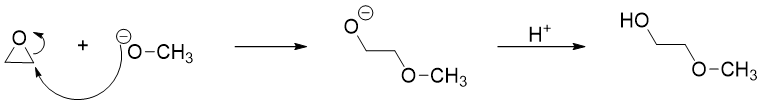
In basic or neutral medium, using unsymmetrical epoxides, the nucleophile attacks on the carbon atom containing high number of hydrogen atoms or containing least number of carbon atoms. This is because the reaction is purely SN2 i.e., SN2 mechanism is commonly followed by least substituted carbon atom. For example,
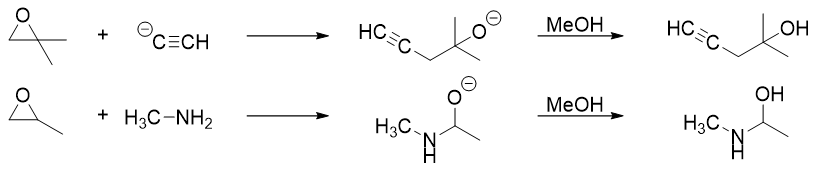
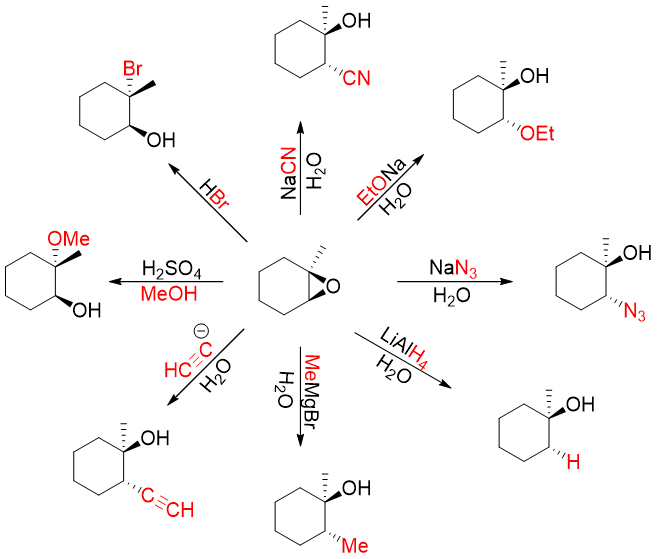
Following is the list of reactions involving opening of epoxide.