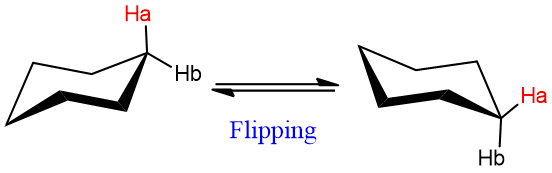
Energy Calculations of Chair Cyclohexane
Energy Calculation of Chair Conformations of Cyclohexane:
Cyclohexane has two major types of conformations; Chair conformation and Boat conformation.
Chair conformation is more stable than boat conformation because it has lower energy due to less steric repulsions as compared to boat conformation.
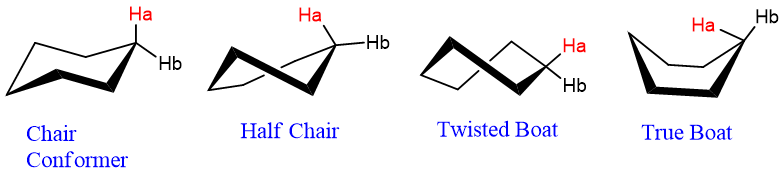
However, cyclohexane exhibits several other conformations while flipping one chair conformation to another chair.

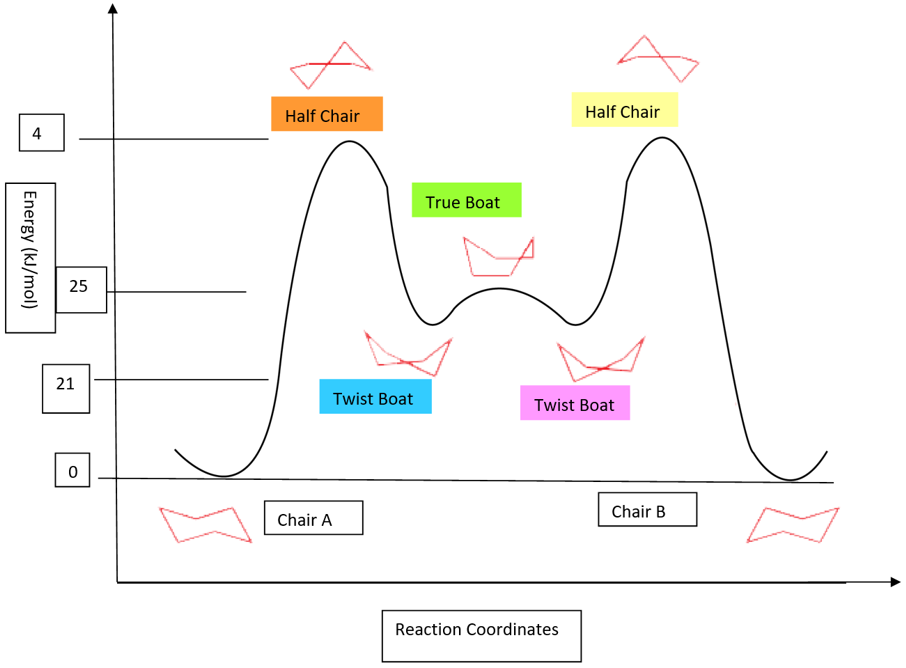
The flipping of these chair conformations passes through the following conformations:
- True chair conformations that have the lowest energy are at the bottom of the energy profile diagram. These have 0 kJ/mol energy.
- Half Chair conformation has the highest energy among all conformations of cyclohexane. It has the maximum repulsion between the bonds and offers the highest torsional and steric strain. It occupies the energy maxima in the energy profile diagram. It has an energy value of about 10 kcal/mol or 4kJ/mol.
- The other conformation that has more energy is boat conformation. It is also not very stable but it has less energy than half chair conformation. It has an energy of about 6 kcal/mol or 25kJ/mol.
- The last conformation is twisted boat conformation, which is more stable than the boat and half chair conformation but still it is less stable than chair conformation. The conformation has an energy of about 5 kcal/mol or 21kJ/mol.
These conformations are shown below;
After flipping from one chair to another, all axial hydrogens become equatorial and vice versa.