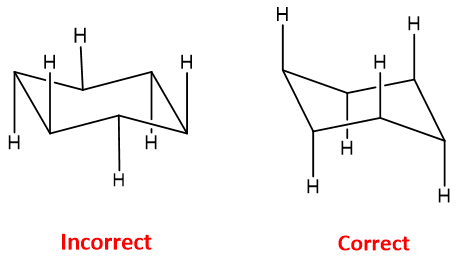
Chair Conformation
Chair Conformation:
The cyclohexane is twisted like a chair form. There are two chair conformations possible both are equal in stability. H.Sachse, In 1890 developed the chair conformations of cyclohexane.
Features of Chair Conformation:
- Chair conformation is the dominant conformation of cyclohexane. It exists more than 99.8 percent because it is the most stable conformation having no torsional and angle strain.
- The C-C bonds in ring are which is very close to the normal tetrahedral angle 109.5. So, due to this there is no angle strain.
- All C-H bonds in the ring are arranged to attain a staggered conformation, due to this torsional strain is also eliminated.
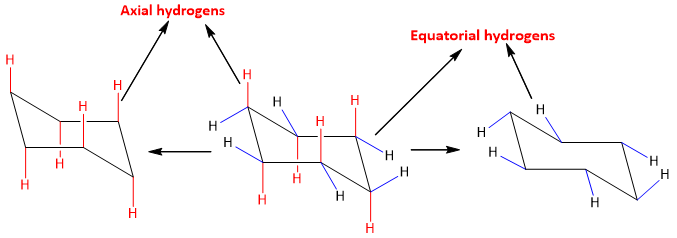
- In cyclohexane, there are two types of hydrogens viz equatorial and axial. Axial Hydrogens Ha, the hydrogens that are present vertically upside or downside. Equatorial Hydrogens He, the hydrogens that are present to some angle along sideways. The equatorial and axial hydrogens are shown below;
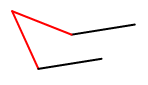
How to draw a chair conformation:
The easiest way to draw a chair conformation is by starting off from one end.

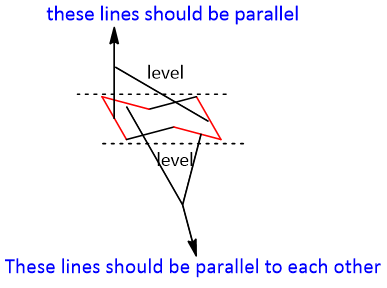
Then the other two lines added joined at one point and the lower joint point should be in parallel with the central parallel lines.
Addition of hydrogens:
Adding axial hydrogens is quite easy. Add hydrogens in a way to make each carbon tetrahedral. Add hydrogens in vertical alignment with alternating up and down positions all over the ring.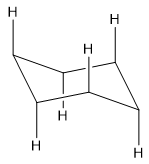
Equatorial hydrogens are quite tricky to add. In order to add hydrogens at the equatorial position, add hydrogens in such a way that the added hydrogens are parallel to two C-C bonds in the cyclohexane ring. The addition of equatorial bonds on the cyclohexane ring is shown below;
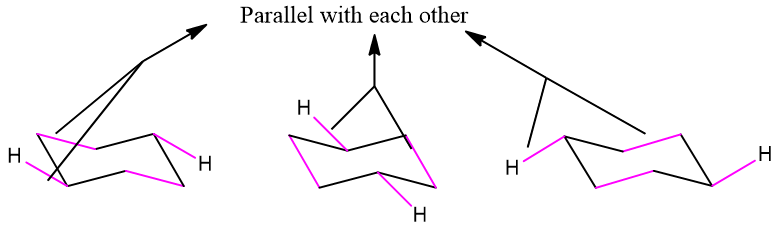
By adding all equatorial hydrogens to the ring an M and W arrangement should appear like the one shown below;
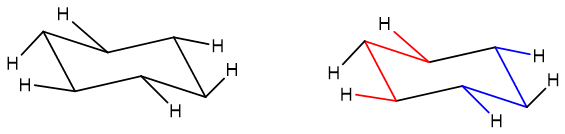
Common mistakes while drawing chair conformation:
Following are some mistakes that can happen while drawing chair conformation:
The chair may have not been drawn incorrectly; the level of the upper points is not balanced. This may happen when the middle C-C bonds in the ring are in a horizontal position rather than tilted.

While drawing equatorial hydrogens, the hydrogens at the middle carbons of the chair have been drawn at the wrong angles. Not making the M and W arrangements.
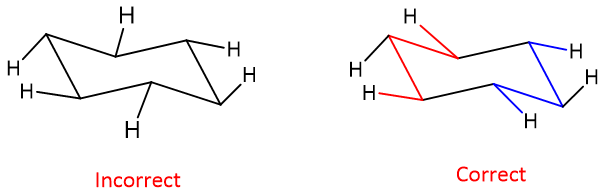
The position of the axial hydrogens has been drawn incorrectly, like hydrogens my drawn on alternative carbons but in the wrong orientation.