Conformations of Cyclohexane
Conformations of Cyclohexane:
Cyclohexane has one six-membered carbon ring. It is a homo-cyclic organic compound with the formula C6H12 (CnH2n).
Like alkanes, cyclohexane also shows different conformations. There are two types of conformations of cyclohexane; Boat conformation and Chair conformation. Both of these are non-planers having zero angle strain.
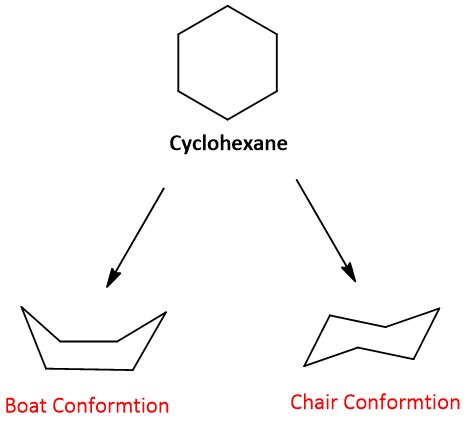
Boat Conformation:
A puckered conformation in which 1 and 4 carbons bent facing each other. It is the high-energy conformation of cyclohexane in which atoms at 1, 2, 4, and 5 are coplanar.
The atoms at 3 and 6 are out of the plane. In this conformation, four C-H bonds eclipsed each other.
The hydrogens at 1 and 4 create torsional strain anda steric interaction also develops between these hydrogens called Flagpole interaction (fp interactions). The hydrogens are called flagpole hydrogens.
In boat conformation, the distance between the two flagpole hydrogens is 1.84 A.
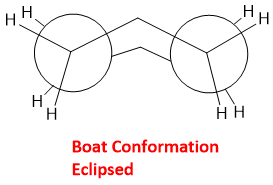
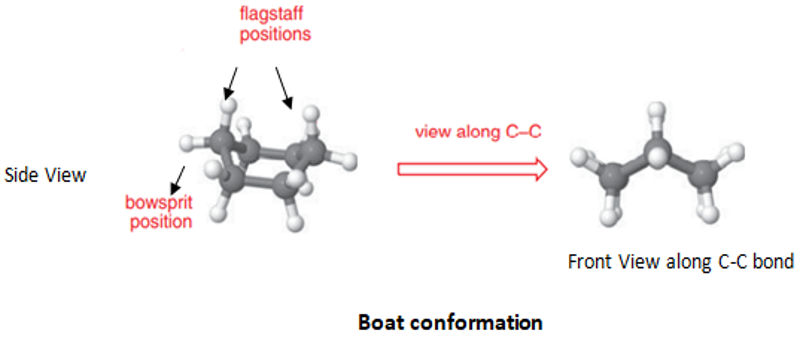
Due to this eclipsing the boat conformation is very unstable; cyclohexane exists in boat conformation to only 0.1 to 0.2 %. This conformation has approximately 25kJ/mol more energy than chair conformation.
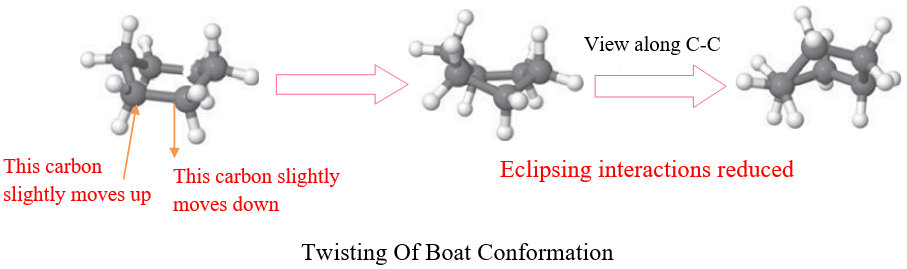
However, this instability can be decreased by twisting the two carbons slightly relative to each other. These twisting results in another confirmation of cyclohexane called Twist-boat conformation. Although this confirmation is lower in energy than boat conformation it is still unstable as compared to chair conformation.
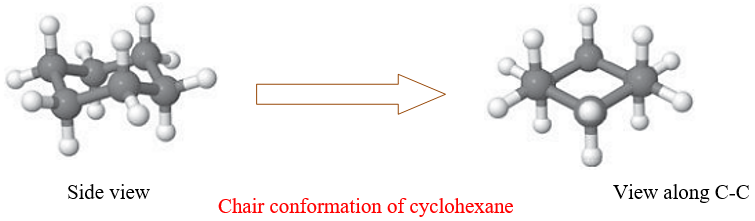
It is the most stable conformation of cyclohexane. All the equatorial and axial hydrogens are staggered, there is no angle strain, no steric hindrance, and a torsional strain is present.
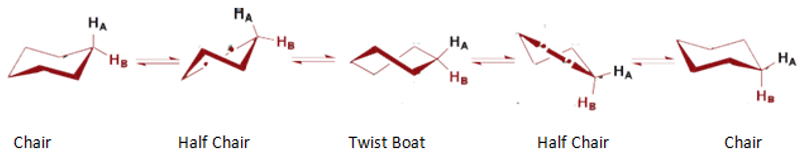
Other conformations of cyclohexane are also possible, which are shown below;