Synthesis of Ethers
Synthesis of Ethers
Simple ethers like diethyl ether are synthesized commercially by reacting alcohols in the presence of sulfuric acid as a catalyst. The reaction occurs via SN2 pathway. This method is limited to primary alcohols because secondary and tertiary alcohols can undergo dehydration reaction via E1 pathway to produce corresponding alkenes.
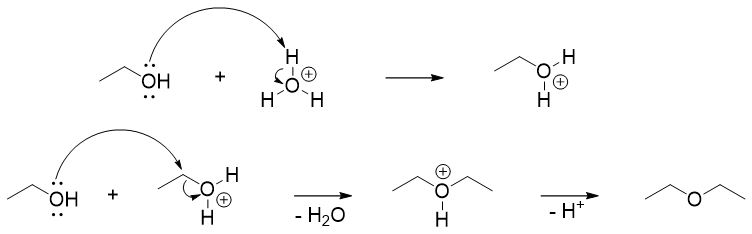
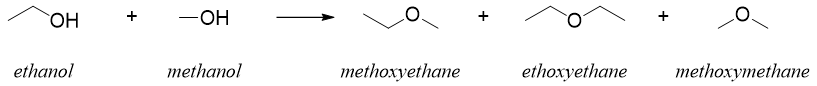
The formation of ethers from alcohols are limited to primary alcohols and it is only preferred to synthesize symmetrical ethers. If two different alcohols are reacted, then the reaction will produce mixture of ethers. For example.
There are other methods employed to synthesize both symmetrical and unsymmetrical single desired ethers. These methods are discussed below.
The Williamson Ether Synthesis:
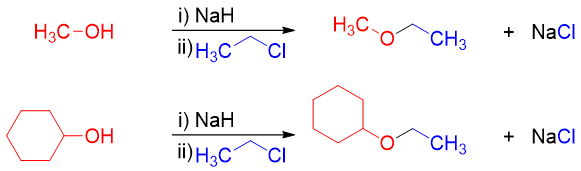
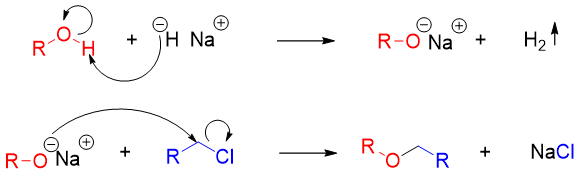
In this method an alcohol is treated with a base to convert it into alkoxide. The resulting alkoxide is further reacted with primary alkyl halide or tosylate to undergo SN2 reaction and produce desire ether.
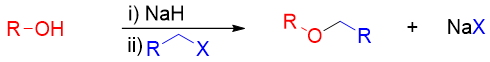
Examples:
Mechanism:
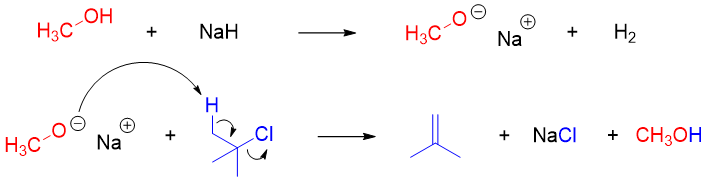
As Williamson ether synthesis is SN2 reaction therefore, it works best for primary alkyl halides and tosylates. Therefore, in unsymmetrical ether synthesis the alkoxide should be of more hindered partner and the alkyl halide should be of less hindered partner. For example, in the synthesis of tert-Butyl methyl ether the alkoxide should be made from tert-Butanol and the alkyl halide should be methyl halide.
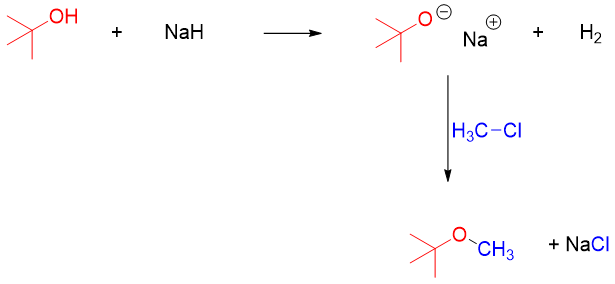
If the order is reversed, then unwanted by products can form due to elimination reaction.
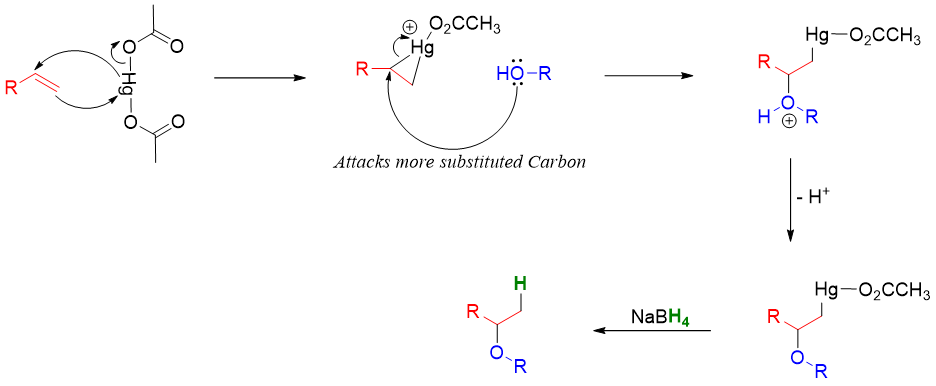
Alkoxymercuration of Alkenes:
When alkene is reacted with alcohol in the presence of mercuric acetate or mercuric trifluoroacetate alkoxymercuration product is formed. The treatment of alkoxymercuration product with NaBH4 breaks the Hg-C bond and yields corresponding ether. This reaction follows Markovnikov’s rule.
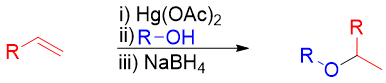
Mechanism: