Ether Cleavage with Acid
Ether Cleavage with Acid
Ethers are one of the unreactive organic compounds. This unreactive chemical property makes ethers widely used as a solvents. Ethers do not react with bases, halogens, dilute acids, or any other nucleophiles. Ethers undergo only one true reaction. They are cleaved into corresponding alcohol and alkyl halide when reacted with strong acids like aqueous hydrogen bromide (HBr) or hydrogen iodide (HI). The resulting alcohol on further reaction with acid produces corresponding alkyl halide.
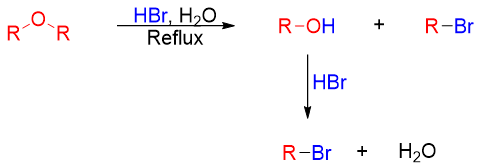
Examples:
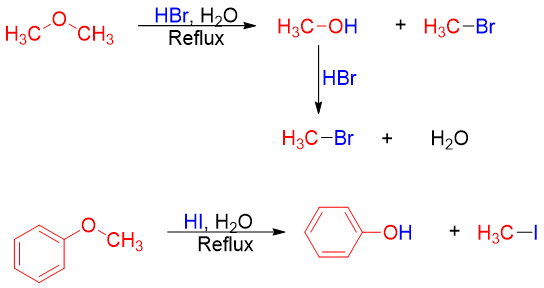
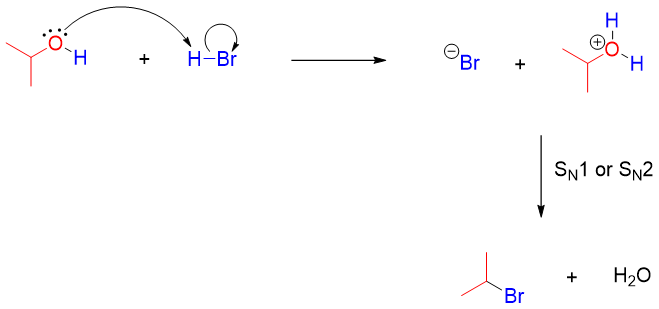
The cleavage of ethers is a nucleophilic substitution reaction, and it can take place either by SN1 or SN2 pathways depending upon the structure of ether. Ethers containing primary and secondary alkyl chains follow SN2 mechanism in which the halide ion attacks the protonated ether at the less hindered carbon. For example.
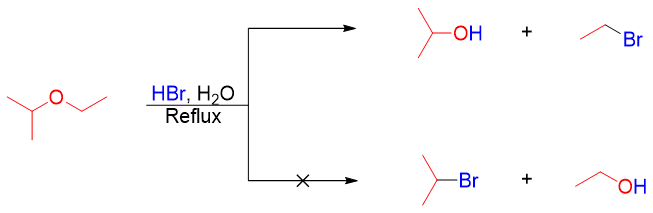
Mechanism:
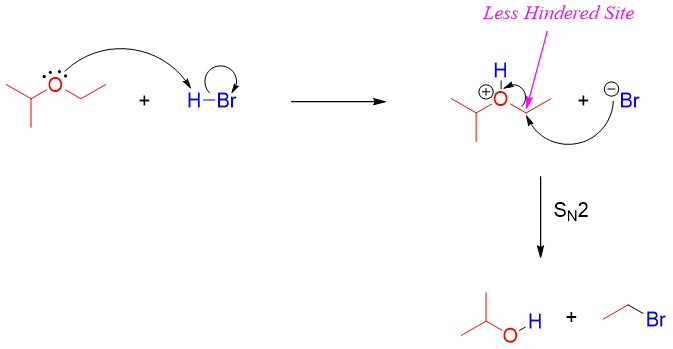
The alcohol produced after the cleavage of ether can further react with acid to generate a good leaving group i.e., H2O. Protonated alcohol reacts with halide ion either by SN1 or SN2 mechanism to produce corresponding alkyl halide.
Phenyl ethers upon cleavage with strong acids cleaves into phenol and corresponding alkyl halide. Phenol does not react further with acid to produce halobenzene as the phenol is sp2 hybridized.
Ethers with tertiary, allylic or benzylic group undergoes cleavage either by SN1 or E1 mechanism. This is due to the formation of stable intermediate carbocation. Such reactions are fast and can take place at room temperatures.
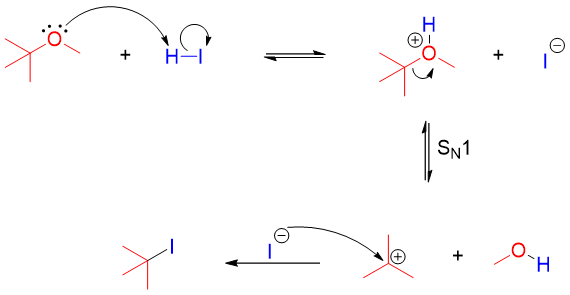
Following example shows the cleavage of an ether containing tertiary alkyl group via E1 mechanism.
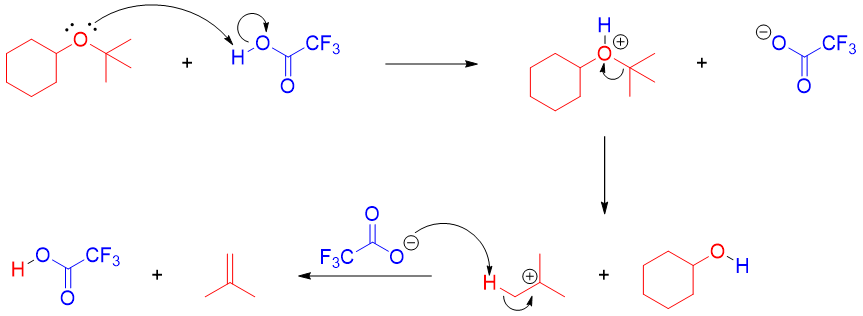
To summarize, an ether containing primary and secondary alkyl groups upon reaction with strong acid will produce alcohol and an alkyl halide from less hindered alkyl group. The alcohol further reacts with acid halide to produce alkyl halide. Whereas ethers containing tertiary alkyl groups will follow SN1 mechanism. The ether will cleave to produce tertiary carbocation and alcohol. The tertiary carbocation can further react to produce alkyl halide (SN1) and alkene (E1).
