Ether Cleavage through Sn1
Ether Cleavage through SN1
The RO- group of ethers and HO- of alcohols have the same basicities as their conjugate acids have pKa values of 15.5 for ROH and 15.7 for HOH. Hence, they are strong bases and are poor leaving groups. To make them react, ethers and alcohols are first activated to undergo nucleophilic substitution reactions.
Ethers are activated by reacting them with strong acids like hydrogen bromide (HBr) and hydrogen iodide (HI). This reaction is slow in nature therefore, it is heated to make it occur at reasonable rate.

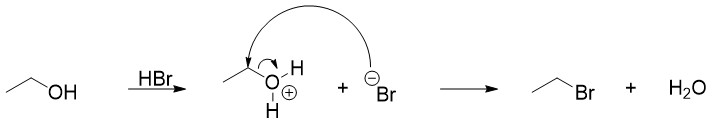
Once the ether is protonated, the structure of ether will decide either the reaction will proceed through SN2 or SN1 mechanism. If the ether contains primary and secondary alkyl groups, then the reaction will proceed through SN2 mechanism. In SN2 pathway the ether is first protonated, then the halide ion will attack the carbon atom containing highest number of hydrogen atoms (less sterically hindered carbon).
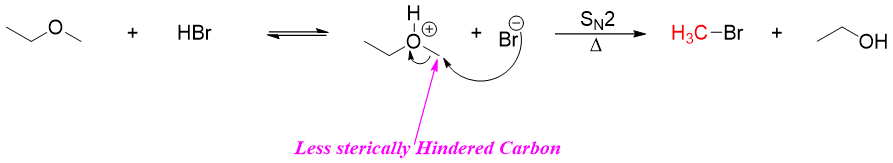
If there is more acid present in the reaction mixture the resulting alcohol will react with it to produce alkyl halide and water.
On the other hand, if the ether contains tertiary alkyl groups, then the reaction will proceed through SN1 pathway. When such ethers are protonated then it is not possible for nucleophile to undergo backside attack due to steric hindrance. In such case the protonated ether cleaves forming an alcohol and a tertiary carbocation. The carbocation reacts with halide ion to produce alkyl halide. The alcohol produced will further react with excess acid to produce alkyl halide.
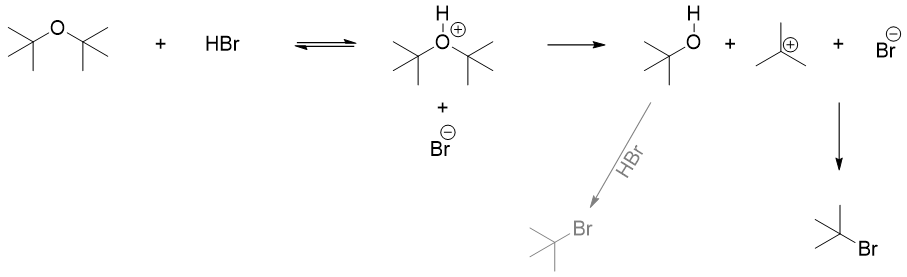
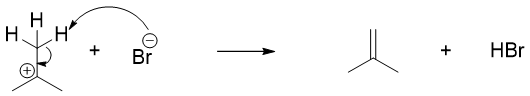
The tertiary carbocation formed in above example can also undergo E1 reaction to produce alkene. Hence, acid cleavage of tertiary ethers also produces elimination byproducts.
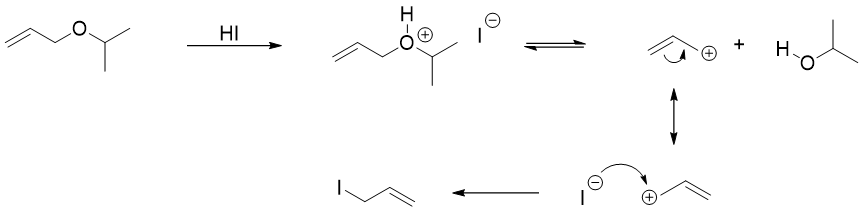
Those substrates which produces stable carbocations cleaves through SN1 pathways. In following example, the allylic group produces resonance stabilized carbocation therefore, it will prefer SN1 mechanism.
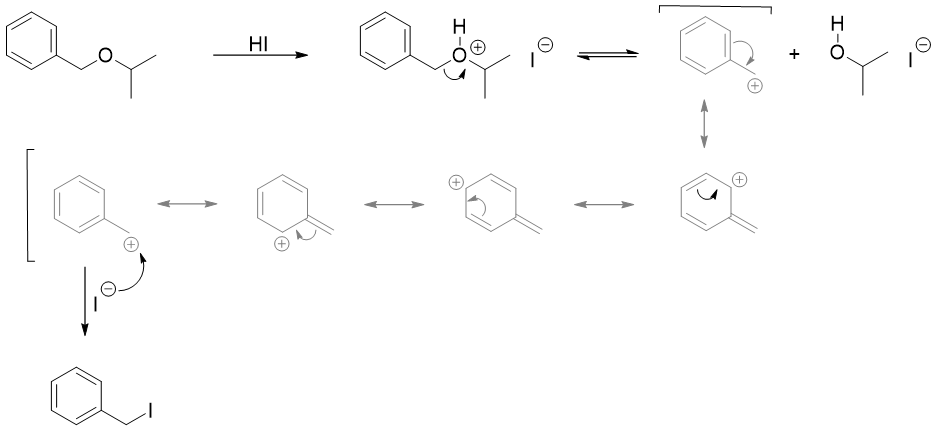
Another example includes the cleavage of benzylic ethers via SN1 mechanism which also produces resonance stabilized carbocation.