
Orgoreview
Question Vault
Welcome to orgoreview question vault. We have 5000 more problems for you to solve.
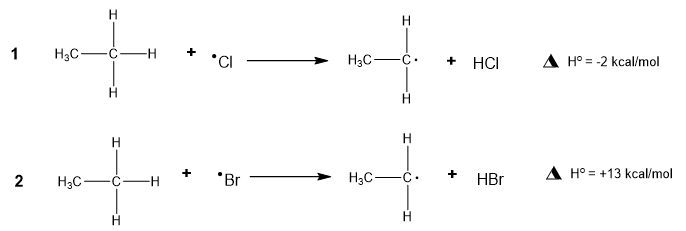
Choose the incorrect statement about the following two reactions.


Welcome to orgoreview question vault. We have 5000 more problems for you to solve.
