
Orgoreview
Question Vault
Welcome to orgoreview question vault. We have 5000 more problems for you to solve.
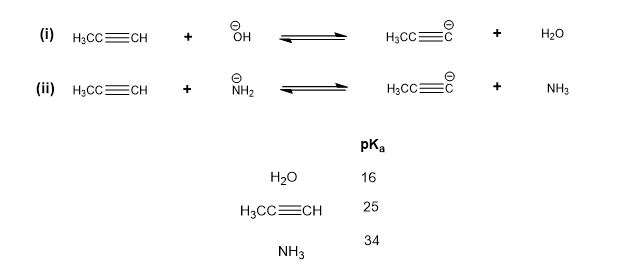
Choose the incorrect statement about the following acid/base reactions involving propyne and its anion propynide.


Welcome to orgoreview question vault. We have 5000 more problems for you to solve.
