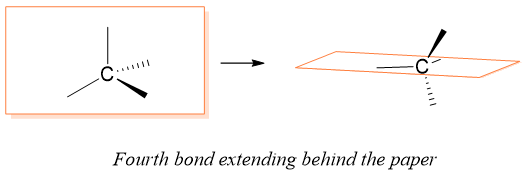
Enantiomers
Enantiomers
Molecules that have same molecular formula, same connectivity patterns but are non-superimposable mirror images of each other are called enantiomers. Consider following generalized molecules with their reflected images in the mirror.
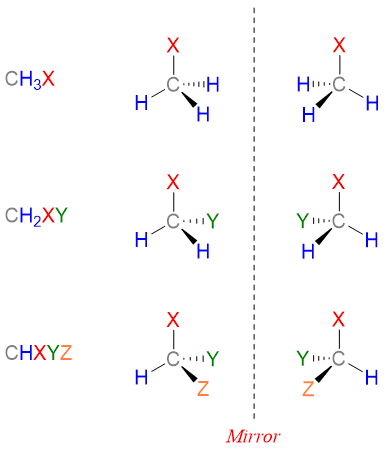
In above figure, molecule CH3X and CH2XY are identical to their mirror images. Both of these molecules can be superimposed on their mirror images as all the atoms can overlap. Whereas the molecule CHXYZ and its mirror image are not superimposable on each other. Hence, CHXYZ has two stereoisomers which are mirror images of each other and cannot be superimposed on each other. This kind of stereoisomers are called enantiomers. Enantiomers are given by those compounds which have chiral centers. Chiral centers are thos centers which is bonded to four different atoms or groups.
Enantiomers can be drawn using Perspective formulas or Fischer projections. Let’s use perspective formula of 2-butanol to draw the enantiomers.
a) First of all, identify the stereocenter in 2-butanol and indicate it by star (*).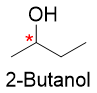
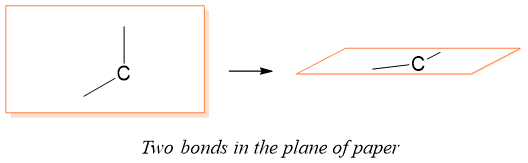
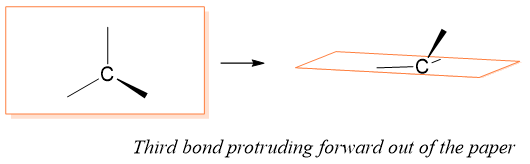
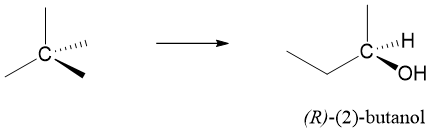
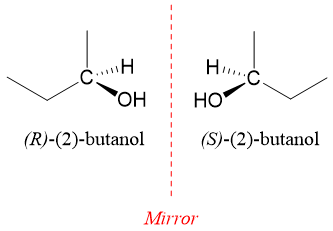
R and S nomenclature of Enantiomers:
The Cahn-Ingold-Prelog (CIP) priority system, rules or conventions are used to name configurations of stereocenters. Each chiral center is given a letter R or S based on its 3-D structure. Two steps are followed to assign name.
1) Assign priority to bonded groups:
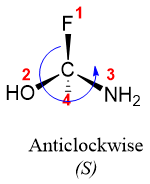
Atoms with highest atomic number directly attached to chiral atoms gets the first priority. For example, in 1-amino-1-fluoro-1-ethanol four different atoms are directly bonded to chiral carbon i.e., H, N, O, and F. Fluorine with atomic number 9 gets the first priority, oxygen with atomic number 8 gets second priority, nitrogen with atomic number 7 gets third priority and carbon with atomic number 6 gets fourth priority.
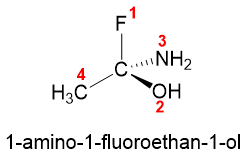
Following are some examples of priority for different atoms attached to chiral carbon atoms.
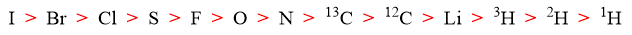
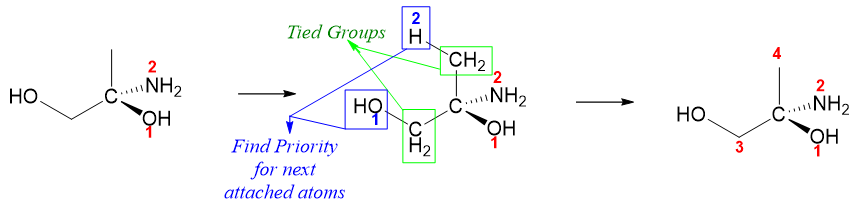
Incase if there is a tie in first step i.e., two directly attached atoms are the same, then use the next atoms attached to tied atoms. For example, in following compound there are two methylene groups attached to chiral carbon. Hence, it’s a tie. We will now look for the atoms attached to these two methylene groups. The greater the priority of next attached atom, the greater the priority of given methylene and vice versa.
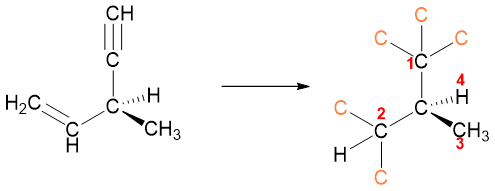
Multiple bonds are treated as the carbon atom is bonded to separate multiple carbon atoms. In double bond the carbon is treated as it is bonded to two carbon atoms while in triple bond it is bonded to three carbon bonds. For example
Once the priority order is assigned, rotate the molecule is such a way that the fourth priority group is pointed backwards.
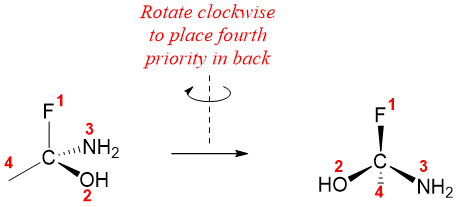
Now, draw circular arrow starting from priority one, going through priority two and ending at priority three. If the arrow is moving clockwise then the configuration is R, if the arrow is moving in anticlockwise direction, then the configuration is S.
Same steps are involved to measure the configuration of the other enantiomer.

