Enthalpy and Entropy
Enthalpy and Entropy
The relationship between equilibrium constant and ΔG is given by the expression
Above expression shows that the direction in which the equilibrium goes depends upon the magnitude and sign of the free energy ΔG. If the value of ΔG is negative, then the equilibrium will favor the product and if the ΔG is very large and negative then the reaction will go to completion. Following table shows variation of Keq with ΔG.
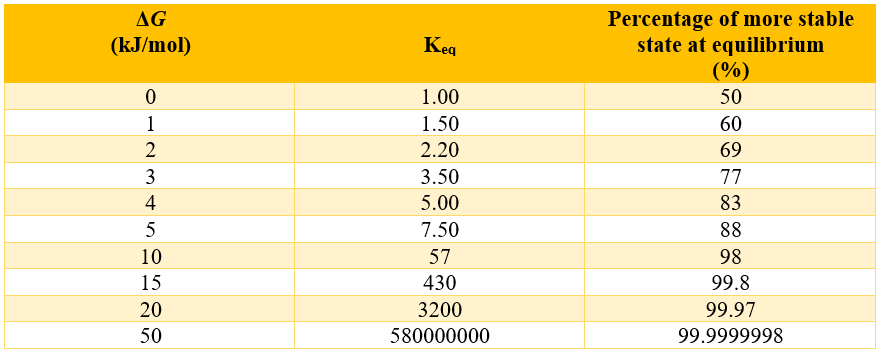
Above table shows that the ΔG value of -10 kJ/mol is enough to get the reaction to completion (i.e., 98%). How the ΔG value corresponds physically, we will introduce another equation.
ΔG = ΔH – TΔS
In above equation H is enthalpy which is the measure of heat and ΔH is the change in enthalpy which measure the heat gained or lost during the chemical reaction. Reactions which have negative ΔH value gives out heat and are called exothermic reactions and reaction with positive ΔH values gains heat and are called endothermic reactions. T is the temperature measured in kelvin (K). Furthermore, S is the measure of disorder in the system and ΔS is the entropy change between the reactants and products. Following table summarizes the effect of changes in Enthalpy, Entropy and Gibbs free energy on spontaneity of a reaction at constant pressure and temperature.
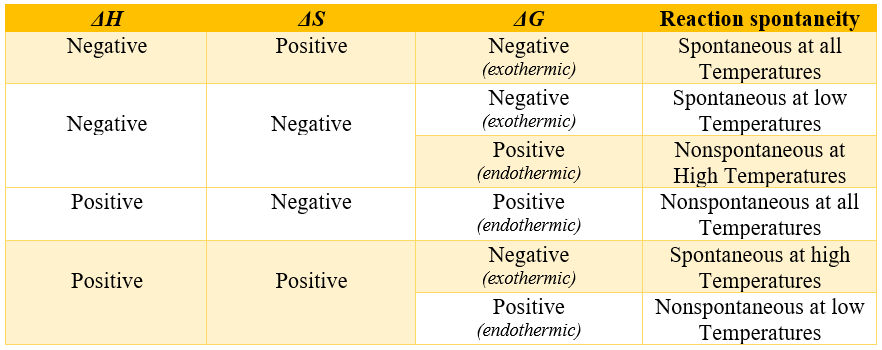
Entropy vs Enthalpy:
The intermolecular formation of hemiacetal by the reaction of ethanol and acetaldehyde at equilibrium is given below.
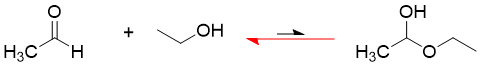
The extent of equilibrium shows that the reaction is not favored in forward direction while, it is favored in backward direction. This is due to enthalpy and entropy.
Enthalpy of C=O (carbonyl bond) in acetaldehyde is about 799 kJ/mol whereas, the enthalpy of two C-O (carbon single bond O) bonds in hemiacetal is (358 × 2) 716 kJ/mol. Thus,
Hence, for above reaction ΔH is small and negative. Furthermore, the entropy of reactants is greater than the products because the reactants side has two moles of molecules while the product side has one mole of molecules.
Thus, the ΔS for above reaction is negative. If the ΔH is small and negative and the ΔS is negative, then the ΔG is positive and the equilibrium of the reaction lies to the left.
On the other hand, in intramolecular formation of hemiacetal the ΔH is again small and negative and the ΔS is no longer negative as the number of molecules in reactants and products are the same hence, the ΔG will be negative and the equilibrium of the reaction lies to the right.

Equilibrium constant vs Temperature:
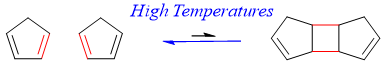
Consider following dimerization reaction of cyclopentadiene.
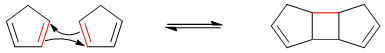
In above reaction two π-bonds reacts to form two new σ-bonds. σ-bonds being stronger than π-bonds have less enthalpies. i.e.
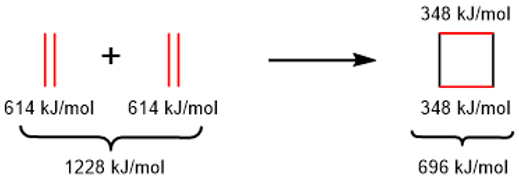
Furthermore, the entropy of the reactants (two molecules) is greater than the entropy of the product (single molecule). Thus, the ΔH is negative and large and the ΔS is also negative so the ΔG is negative at low temperatures and the equilibrium will shift to the right at low temperatures. i.e.

If the temperature is increased, then the ΔG value will become positive and the equilibrium will shift to the left. i.e.