Bond Dissociation Energy
Bond Dissociation Energy
In a chemical reaction both the breakdown and formation of bonds take place. It is necessary to understand the energies required to form bonds and energies released upon the formation of the bonds. Bond dissociation energy or bond dissociation enthalpy is defined as the energy required to cleave a bond homolytically at 298 K.
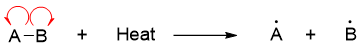
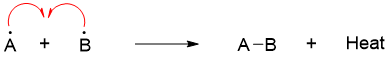
Similarly, when the two radicals combine to form molecule AB, then according to first law of thermodynamics the energy equivalent to the bond dissociation energy is released.
The energetics of wide range of reactions can be calculated in three steps.
1) Bond energies of all the bonds present in reactants are added together. i.e., ΣHreactants
2) Bond energies of all the bonds present in products are added together. i.e., ΣHproducts
3) The energy change is then calculated by using following equation.
ΔH = ΣHreactants ˗ ΣHproducts
Following table shows bond dissociation energies of different bonds (highlighted Red).
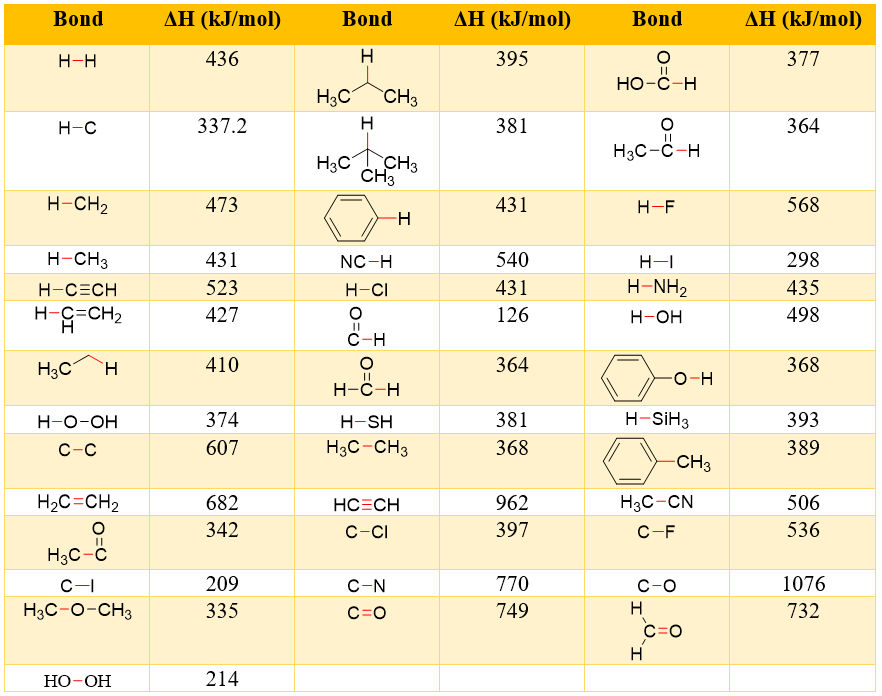
When hydrogen gas reacts with chlorine gas to produce hydrogen chloride gas. The total energy change is calculated as.
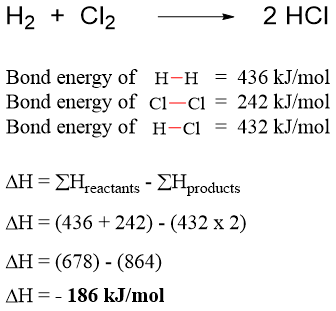
The negative sign shows that the energy is released therefore, the reaction of hydrogen gas and chlorine gas is an exothermic reaction.
Similarly, the energy change in decomposition of hydrogen chloride gas can be calculated as.
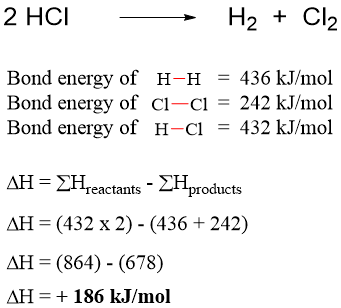
In this case the amount of energy is same, but it has a positive sign. This shows that the reaction is endothermic. Thus, to decompose HCl we should add energy into the system.
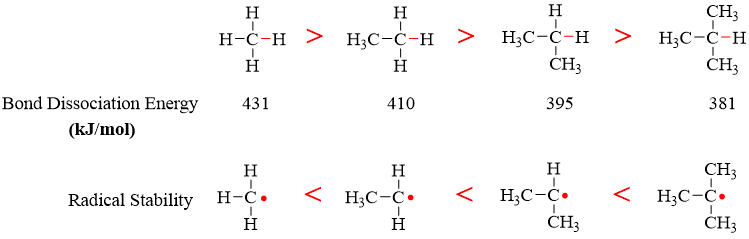
The magnitude of bond dissociation energy reflects the strength of a bond. We already know that tertiary carbon radicals are more stable that secondary and primary carbon radicals. This is due to bond dissociation energies.
Another example involves the usage of peroxides in hydrohalogenations of alkenes. In the presence of peroxide the alkene gives anti Markovnikov product. As the peroxide and hydrogen halide both are present, and both can cleave homolytically but due to lower bond dissociation energy of -O-O- bond the peroxide cleaves homolytically at little heat or light.
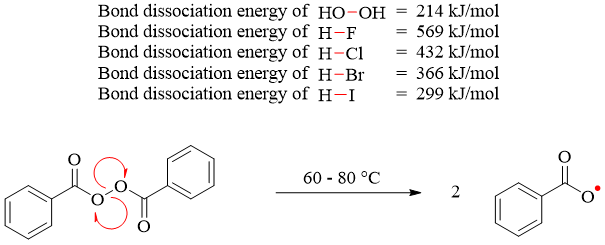
The peroxide radical further propagates the hydrohalogenation reaction by forming free radicals of hydrogen halides.
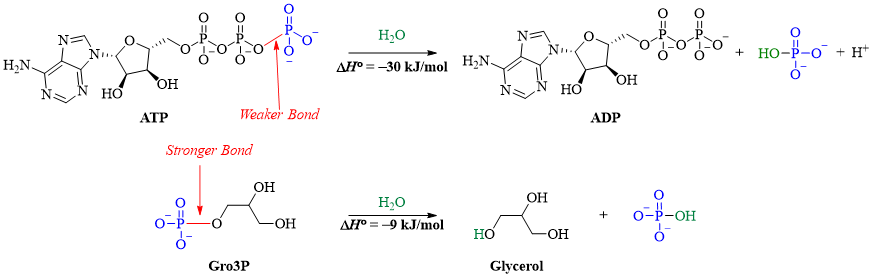
Another important reaction includes the energy provided by ATP. The ATP has weak bonds with low bond dissociation energies. These weak bonds break when small amount of energy is provided, and a large amount of energy is released when new strong bonds are formed. For example, hydrolysis of organic phosphates gives off less heat while, ATP gives out more heat when hydrolyzed. This is because the bond in organic phosphates is stronger thus requires more energy to break it.