Diels Alder
Diels-Alder Reaction
Pericyclic Reactions:
This is special class of organic reactions in which two reactants with π-electron systems undergo a reaction to form a cyclic product. The characteristic feature of these reactions is the formation of cyclic and concerted transition states in which all the π bonds overlap in a cyclic manner. Following are several types of Pericyclic Reactions.
- a) Electrocyclic Reactions
- b) Sigmatropic Reactions
- c) Cycloaddition Reactions
- d) Group Transfer Reactions
- e) Dyotropic Reaction
- f) Cheletropic Reactions
In this lab Cycloaddition Reaction is utilized to form the desired product.
Cycloaddition Reactions:
In cycloaddition reactions two or more unsaturated compound (or side chains of the same molecule) undergo addition to form a ring system with decrease in number of π Bonds.
One example of cycloaddition reaction is Diels Alder Reaction.
Diels Alder Reaction:
Two German scientists Otto Diels and Kurt Alder reported this reaction in 1928. Diels and Alder were awarded with Noble Prize in Chemistry in 1950. In this type of reaction, a six-membered ring compound is formed by reacting an alkene (dienophile) with 1,3-diene (diene).
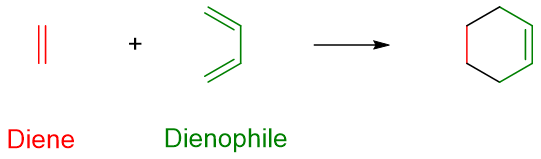
This reaction has many applications and is particularly useful in synthetic chemistry as the product is a cyclic compound which is present in many biologically important compounds like Steroids, Prostaglandins, Reserpine, Taxol, Tabersonine etc.
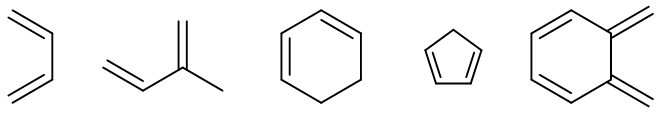
The diene in these reactions is normally electron rich in nature and contains electron donating groups. Few examples are as follow.
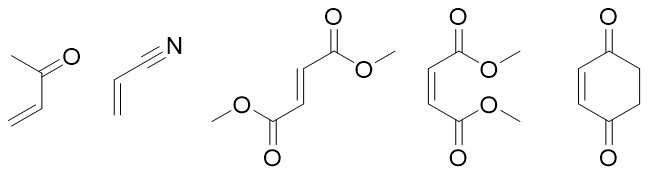
While the dienophiles contain electron withdrawing groups and are electron deficient. Few examples areas follow.
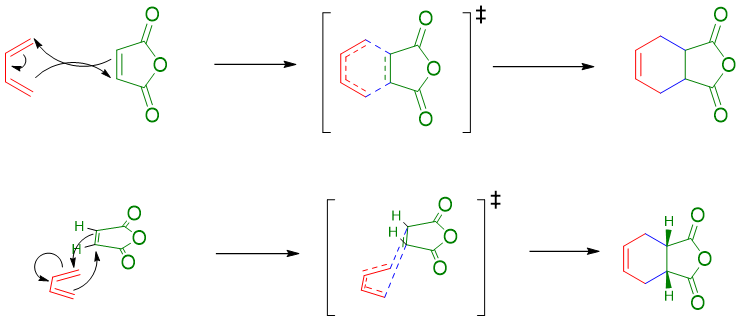
Mechanism:
The mechanism of Diels Alder reaction is single step reaction with concerted type transition state. Also, many other plausible mechanisms are proposed involving free radicals. The simple arrow-pushing and concerted mechanism is as follow.
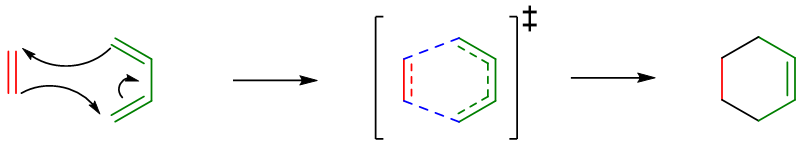
Regioselectivity of Diels Alder Reaction:
Regioselective reactions are those reactions in which two products with different ratios are formed and both occupying various positions in both products. For example, in given reaction two reactants are combining to give two distinct products one para substituted while, the other meta substituted.
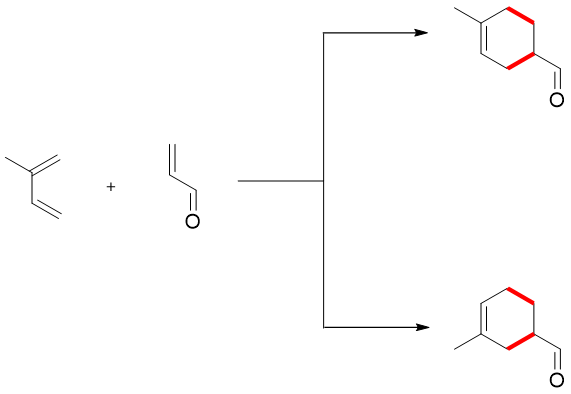
1-substituted dienes when reacted with unsymmetrical dienophile favors the 1,2-product and not the 1,3-product.
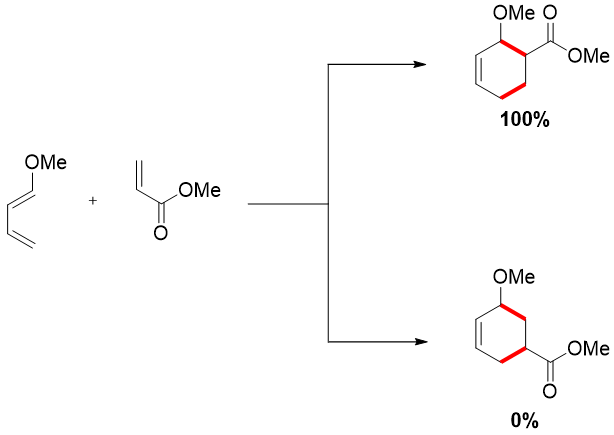
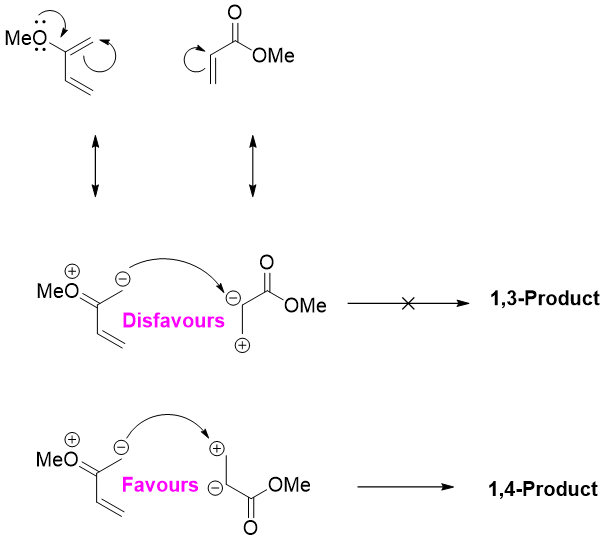
2-substituted diene when reacted with unsymmetrical dienophile favors 1,4-product and not 1,3-product.
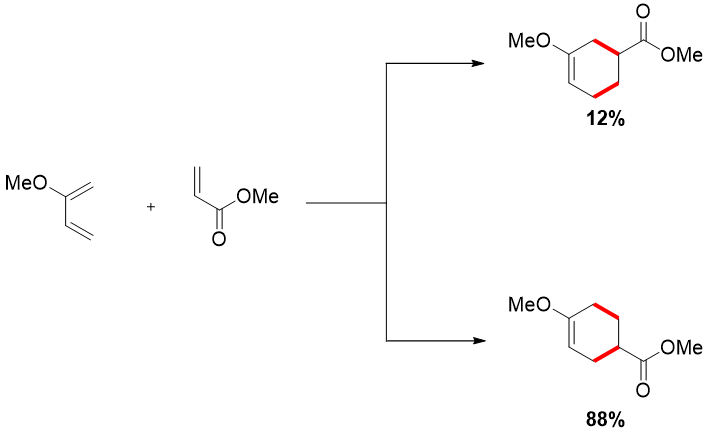
The reason for the difference in regioselectivity depicted below. Drawing the resonance forms of dienes and dienophiles, one can easily understand that the two same charges repel each other making the 1,3-product negligible to form.
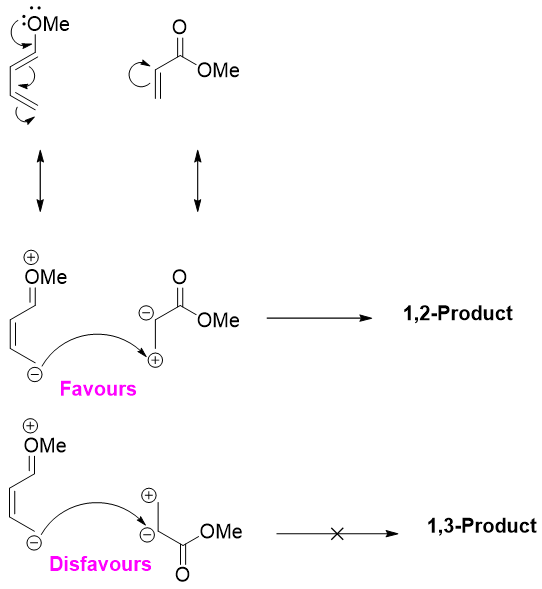
Same factor explains the dominance of 1,4-product in 2-substituted diene with unsymmetrical dienophile.
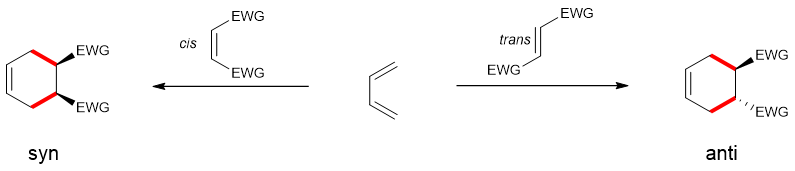
Stereospecificity and stereoselectivity:
Diels Alder Reactions are stereospecific reactions in which the stereochemistry of the products is totally controlled by the stereochemistry of the reactants. The stereo of reactants is retained in the products as shown.
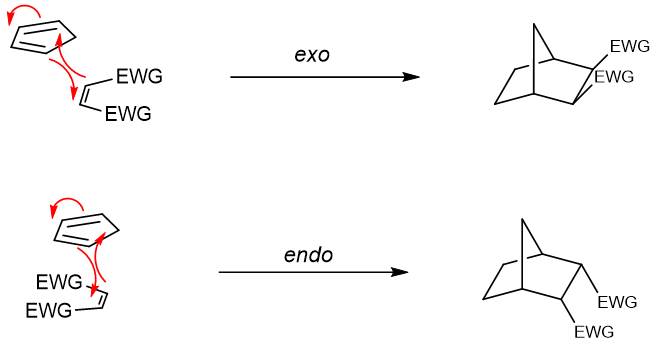
Diels Alder reactions are also stereoselective, where the ratios of stereoisomers formed as a final product depends on the path adopted by the reactants. The reactants can react in an “endo” or an “exo” fashion giving different stereoisomers as shown in following example.
Mechanism of Reaction of Butadiene with maleic anhydride: