Cope Rearrangement
Cope Rearrangement
Sigmatropic Rearrangements:
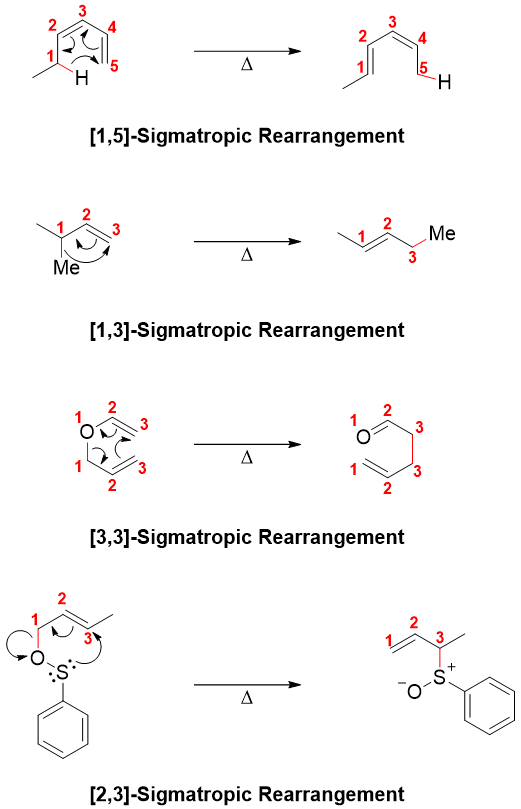
Those arrangements in which the sigma (σ) bond in the reactant breaks and a new sigma bond forms with the rearrangement of pi (π) electrons. Following are some examples of sigmatropic rearrangement reactions.
Cope Rearrangement:
Cope rearrangement is the [3,3] sigmatropic rearrangement. The reactant in Cope rearrangement is 1,5-diene and the product is regioisomeric 1,5-diene. This rearrangement involves the formation of six-membered ring transition state therefore, the reaction follows suprafacial pathway.
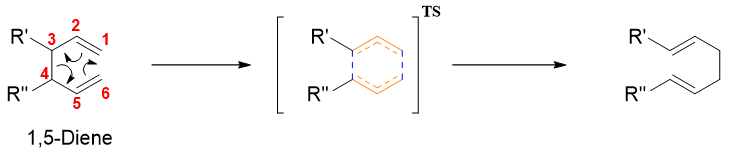
In above reaction two π bonds break and two new π bonds form with the breakdown of one sigma bong between C3 and C4 and formation of new sigma bond between C1 and C6. The Cope rearrangement reaction is a reversible reaction. The equilibrium will shift to the side having more substituted alkene (Zaitsev’s rule). Hence, in above reaction the equilibrium will shift to right side as the product contains more substituted alkene.
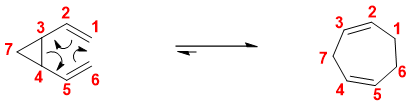
Some examples of Cope rearrangement reactions are irreversible in nature. These reactions involve reactants having large ring strains therefore, the product does not convert back to reactants. For example.
Another example of Cope rearrangement involves formation of enol from rearrangement of 3-hydroxy substituted 1,5-diene. The enol can further tautomerize to delta epsilon unsaturated carbonyl compound.
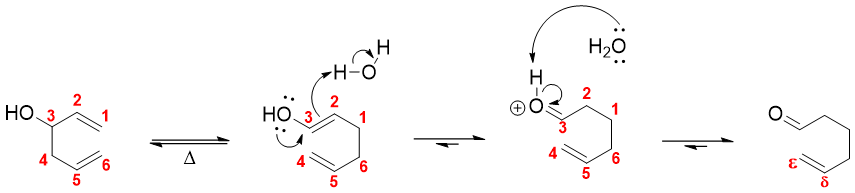
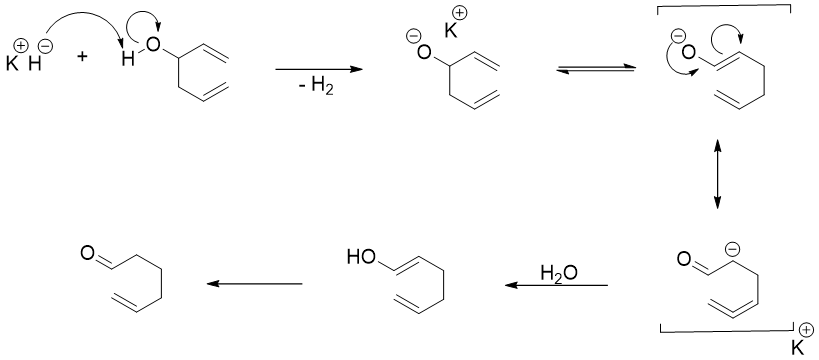
The rate of oxy-Cope rearrangement reactions progresses more rapidly when the hydroxyl group is deprotonated with KH. The rate of reaction 1017 times faster and can be performed at room temperature.
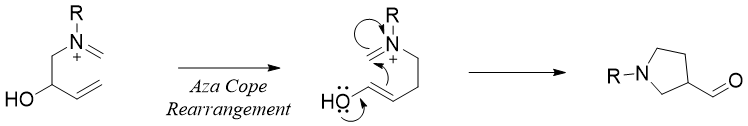
Cope rearrangements can also take place for nitrogen substituted 1,5-dienes. Following are examples of Aza-Cope rearrangements of 1,2, 3 nitrogen substituted 1,5-dienes.
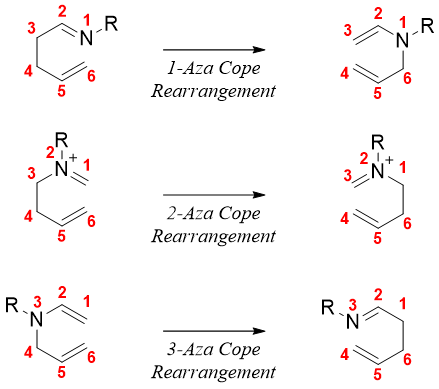
The aza-Cope rearrangement reactions are often utilized in forming nitrogen containing ring systems. For example,