E2 Reaction and Characteristics
E2 Reaction and Characteristics
Elimination reactions
Elimination reactions convert saturated compounds into unsaturated ones and several reactions can achieve this change such as dehydration, dehalogenation, dehydrohalogenation, etc. In this section, we will limit our discussion to dehydrohalogenation reactions which are exhibited by alkyl halides under the influence of bases. For example:
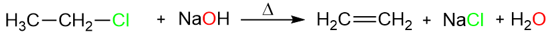
Like substitution reactions, elimination reactions can take place via two types of mechanisms: E1 and E2. This section will discuss the E2 mechanism with its characteristics.
E2 Reaction Mechanism
There are some similarities that E2 reactions share with SN2 reactions. The molecule on which elimination takes place is called the substrate, while the group which is expelled by the incoming base is called a leaving group. However, instead of a nucleophile pushing the leaving group by attaching the carbon atom that bears the halogen atom, the base removes a β-hydrogen, creating a double bond and pushing the halide leaving group. An α-carbon is the one that is directly attached to the leaving group and all the hydrogens on α-carbon are called α-hydrogens. The carbon atom that is next to the α-carbon is called the β-carbon and bears the β-hydrogens.
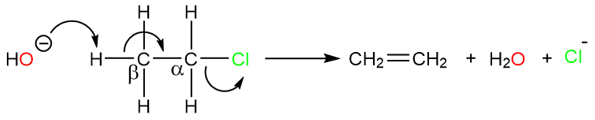
E2 Reaction Kinetics
Just like in an SN2 reaction, the bond breaking and bond formation takes place simultaneously in an E2 reaction. In other words, the reaction mechanism is concerted and takes place in a single step.
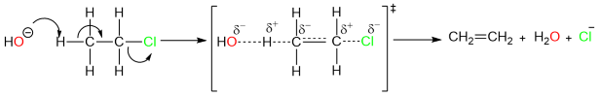
As can be seen here, the base and the substrate are all part of a bimolecular transition state. The reaction is second order and the rate of the reaction depends upon both the alkyl halide (substrate) and the base.
Rate = [RX][B-]
Stereochemistry of the E2 Reaction
E2 reactions are regioselective and stereoselective. The regioselectivity of these reactions will be discussed in a later section and this section will only focus on the stereoselectivity of the E2 reaction. A stereoselective reaction is a reaction where the stereochemistry of the product is dictated by the stability of the transition state.
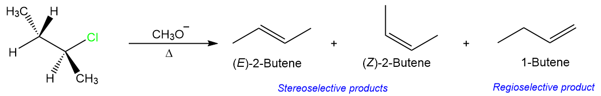
The regioselectivity in the above reaction is due to the presence of two β-hydrogens. The product formed in this reaction is mostly trans ((E)-2-butene) but some about of the cis isomer ((Z)-2-butene) is also formed. The observed stereoselectivity is the result of a lower energy transition state for the trans isomer than the transition state for the cis isomer.
The Antiperiplanar Requirement
Electronic and steric factors dictate the stability of the transition state. β-hydrogens can either be antiperiplanar or synperiplanar. The base only takes the β-hydrogen that is antiperiplanar to the halide leaving group. In the reaction shown above, the substrate has two β-hydrogens, therefore the sigma bond between α-carbon and β-carbon can rotate to form different transition states. Each transition state can have only one β-hydrogen antiperiplanar to the halide leaving group.
The transition state that leads to the formation of the trans isomer is:
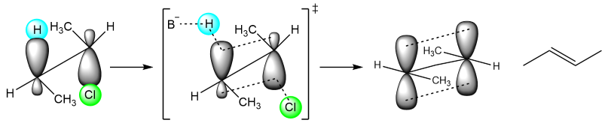
Now, the transition state leading to the cis isomer is:
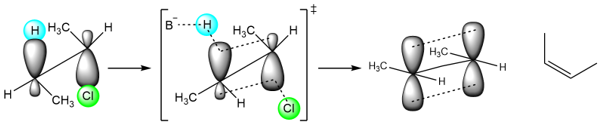
It can be seen that the transition state that leads to the trans isomer is more stable because the two methyl groups are on opposite sides. In the second transition state, the two methyl groups, are on the same side. This creates an increase in the steric hindrance of the transition state. The transition state is destabilized, and the cis isomer is formed in a smaller amount.
Stereospecific E2 Reactions
Stereospecific reactions are those reactions where the stereochemistry of the product is only dependent on the initial stereochemistry of the reactant. The stability of the transition state does not matter.
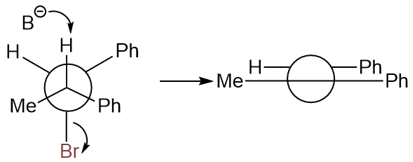
The following alkyl halide only yields the trans product.
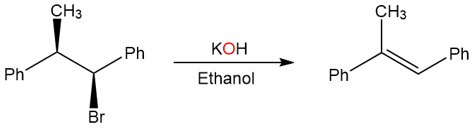
The reason behind the formation of only the trans product is the following antiperiplanarity:
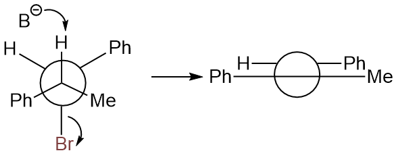
On the other hand, the following substrate produces only the cis product.
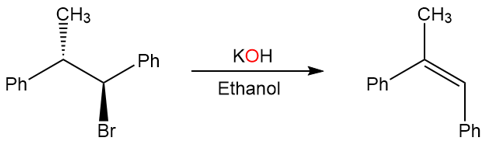
The antiperiplanarity of this reaction is shown here:
Factors Affecting Rate of E2 Reaction
The rate of an E2 reaction depends upon various factors, which are discussed below:
Nature of the Substrate (Steric Hindrance as a Factor)
Steric hindrance is an important factor in determining the E2 reactivity of an alkyl halide substrate. The E2 reaction often competes with SN2 reaction. Since the nucleophile approaches the backside of the carbon that bears the halogen atom, the carbon atom should not be accessible for an E2 reaction so that the base preferably attacks the β-hydrogen. In general, the trend of substrate reactivity in E2 reactions is:
3° > 2° > 1°
Another reason why tertiary substrates react faster than secondary and primary substrates in E2 reaction is that the reaction results in the formation of more substituted alkenes, which are more stable.
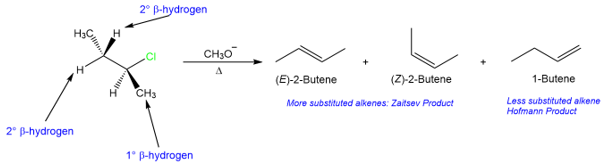
We will this in more detail in a later section (Zaitsev Vs. Hofmann).
Strength of the Base (Nucleophilicity Vs. Basicity)
The strength of the base has a great effect on the rate of E2 reaction. Stronger bases result in faster reaction rates. Negatively charged bases are the best choice for E2 reactions. The most common examples of bases used for E2 reaction are hydroxide (OH−), amide (NH2−), and alkoxides (RO−) such as methoxide or tert-butoxide.
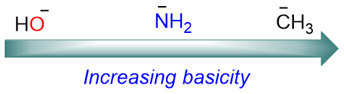
Additionally, not all negatively charged bases are equally effective in E2 reactions. For example, basic strength increases with decreasing electronegativity: NH2− is more basic than OH−. Basic strength decreases down a group: F− is the most basic among all halides while iodide (I−) is the most nucleophilic.
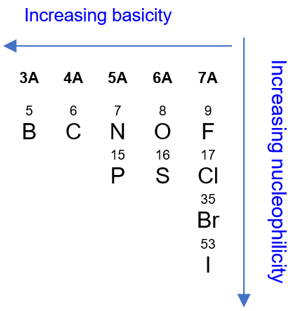
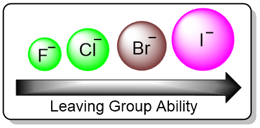
Effect of the Leaving Group
In an E2 reaction, a good leaving will increase the rate of the reaction. Since the rate-determining step involves the base and the substrate along with the halide leaving group, a weaker bond between the carbon and the leaving group results in an easier and faster elimination reaction. In the case of halogens, the leaving group ability increases down the group due to the increasing atomic radii and increasing polarizability of the atom.
Effect of the solvent
The rate of an E2 reaction depends upon the strength of the base. Stronger bases result in faster elimination reactions. The best solvents for E2 reactions are polar aprotic solvents while polar protic solvents are the worst solvents for these reactions. This is because polar protic solvents solvate (or make hydrogen bonds) with the base and thus decrease its basicity. Polar aprotic solvents increase the basicity because the base is “free” or “naked” in the solution.
Effect of Temperature
Elimination reactions are favored at higher temperatures while substitution reactions are favored at lower temperatures. The explanation for this lies in the concept of entropy.
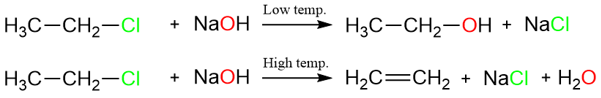
Entropy increases with increasing temperature. As can be seen in the two equations above, an elimination reaction results in a higher molecular degree of freedom (i.e., more atoms/ions produced = more random movements). In other words, an elimination reaction is entropy-favored, so its rate is increased at a higher temperature.
