Free Radical Halogenation
Free Radical halogenation:
The replacement of the hydrogen atom of alkane with the halogen atom is called halogenation. The halogenation takes place with the free radical mechanism. This takes place in the presence of sunlight.
Chlorine and bromine give moderate reactions. Fluorine reacts violently and it gives a mixture of products like various fluorinated alkanes, and hydrogen fluoride.
Iodine also does not give this reaction because its reaction is slow and reversible. HI formed in the reaction which is a strong reducing agent. It gets oxidized back to molecular iodine and alkane.
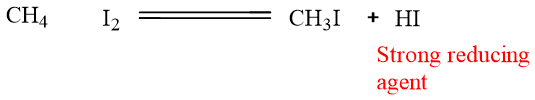
The free radical mechanism includes three steps;
- Initiation:
- Propagation
- Termination
Initiation:
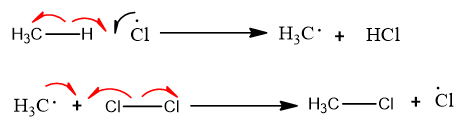
It is the first step. In this step free radicals are formed by the homolysis of neutral reactants.

Propagation:
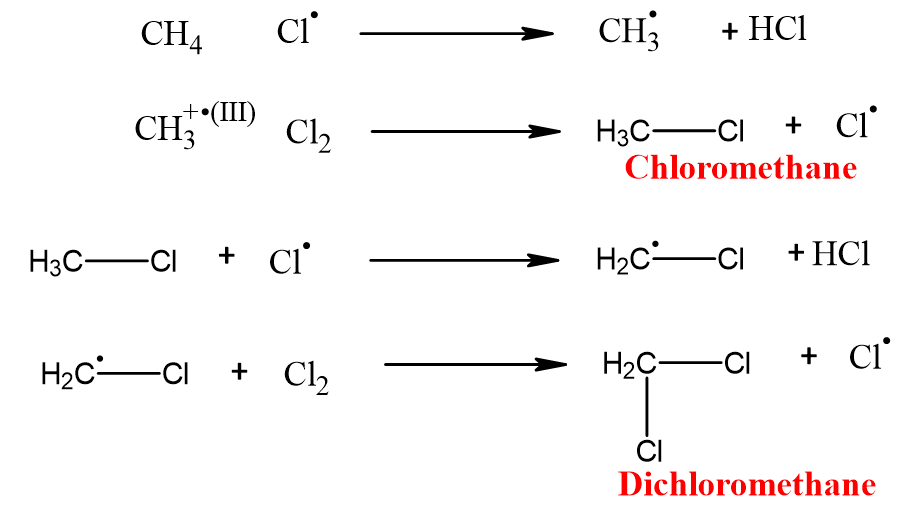
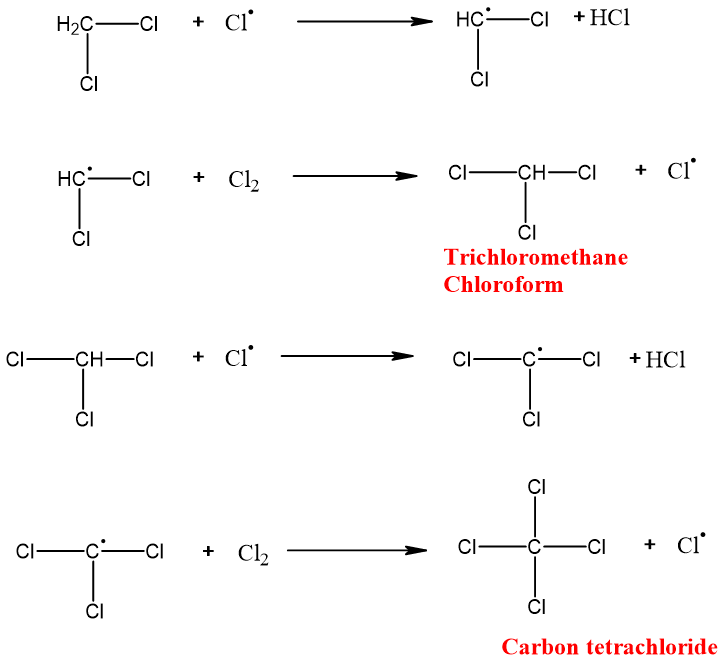
It is a continuous step in which a free radical reacts with the alkane. An alkyl-free radical and neutral (HX) molecule is formed.
The alkyl free radical formed reacts with another halogen molecule. Alkyl halide and halogen-free radicals are formed.
The reaction goes on until termination.
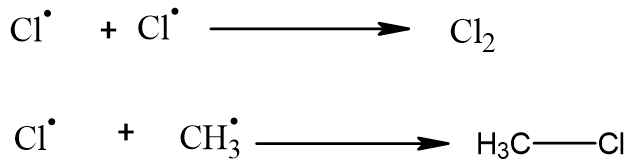
Termination:
Reaction ends when chlorine free radical reacts with alkyl free radical and results in the formation of a neutral product. No free radical is formed.
The halogenation of alkanes takes place until all hydrogens are replaced by the halogen atom. This extent of halogenation depends upon the amount of halogen and light.