Determining the Stereochemistry of Radical Halogenation
Determining the stereochemistry of radical halogenation:
The halogenation that occurs via a free radical mechanism is called radical halogenation. The halogenation of alkanes takes place through the free radical mechanism. In the halogenation of alkanes like propane and higher alkanes, there is the possibility of forming more than one radical.
The single halogenation of methane and ethane gives the same product.
The halogenation of propane can result in more than one product due to the formation of more than one type of free radicals. Propane has two types of hydrogens; primary hydrogens and secondary hydrogens. The hydrogens of two methyl’s (CH3’s in propane) are primary and the hydrogens of the middle CH2 group are secondary. Due to these two types of hydrogens, two types of free radicals are possible to form i.e., primary free radical and secondary free radical.
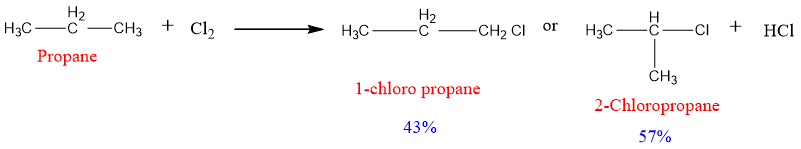
The mechanism that leads to both products can be written as;
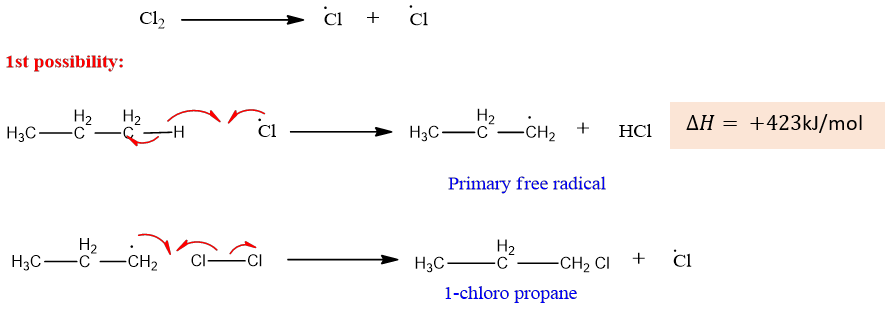
The first possibility is the removal of hydrogen from either of the two methyl groups. This results in the formation of a primary free radical that reacts with chlorine to form 1 chloropropane.
The second possibility is the removal of hydrogen from the CH2 group which results in the formation of a secondary free radical.
The formation of secondary free radical is given as;
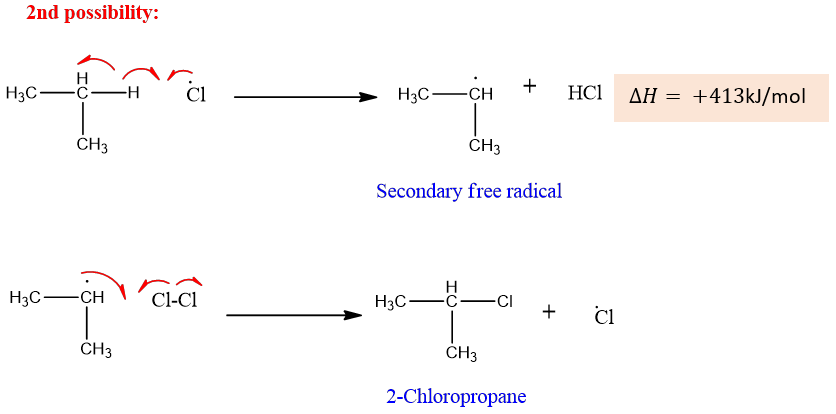
The secondary free radical is more stable than the primary free radical which results in the formation of the major product via the secondary free radical.
The reason for secondary free radical stability over primary free radical is given as;
Hyperconjugation and inductive effect:
Secondary free radical is more stable due to more hyperconjugation and more inductive effect as compared to primary free radical. In secondary free radicals, two alkyl groups are present and have an electron-donating inductive effect, therefore stabilizing the free radical. Whereas in primary free radical only one alkyl group is present. Therefore, the inductive effect is lower in primary free than secondary free radical.
Another effect called hyperconjugation is also present and responsible for the stability of secondary free radicals over primary free radicals. It is the delocalization of the sigma bond (C-H) as the pi bond delocalizes over the conjugated system. The more hydrogen atoms present in the compound more hyperconjugation it will have. The more hyper-conjugated structures a compound has, the more stability it possesses. Secondary carbon has more C-H bonds attached to it, it is more stabilized. In free radicals, the electron density of the C-C and C-H bond overlaps with the pi orbital of free radical carbon.
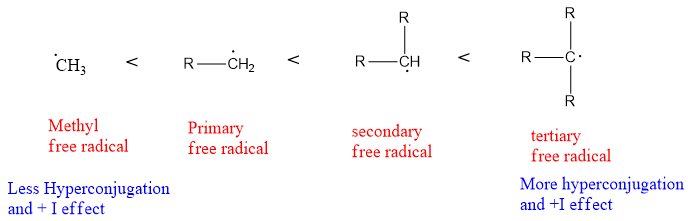
Bond Dissociation energy of C-H Bond:
Experimental data show that the breaking of the C-H bond from a primary carbon requires more energy than secondary carbon. Braking of the C-H bond of a primary carbon requires 10kJ/mol more energy than the secondary C-H bond.
Due to these reasons, selectivity is present among the products of the halogenation of alkanes. That product will always form which will be resulted from stable free radicals.
Consider another reaction:
There is another selectivity that exists between halogens i.e., bromination of alkanes is more selective than chlorination. It is due to the reason that bromine is selective and less reactive than chlorine.
The formation of free radical in chlorination with alkanes is exothermic and bromination is endothermic. In the energy profile diagram, it is shown that in exothermic reactions transition state is closer to reactants than to products.
Whereas, in the endothermic reactions transition state is closer to products than to reactants. It can be concluded that in bromination (endothermic reaction) the transition state is closer to products i.e., toward free radicals. So, for this reaction stability of the product is more important and only one stable product will be formed.
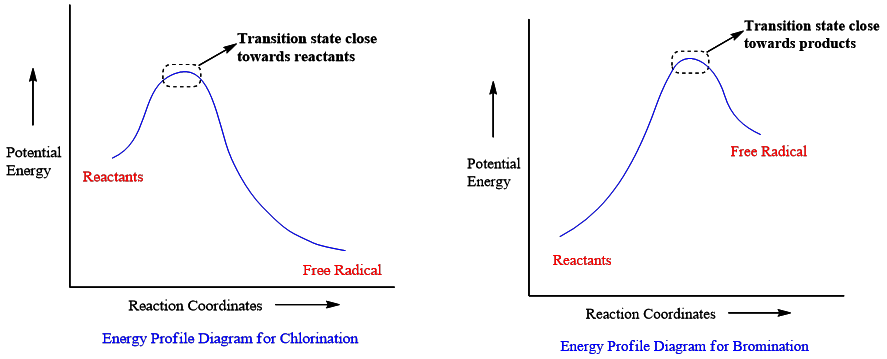
On the other hand, in the case of chlorination free radical character (product) is not pronounced and dominating. So, the stability of the transition state does not matter and chlorination gives all possible products.
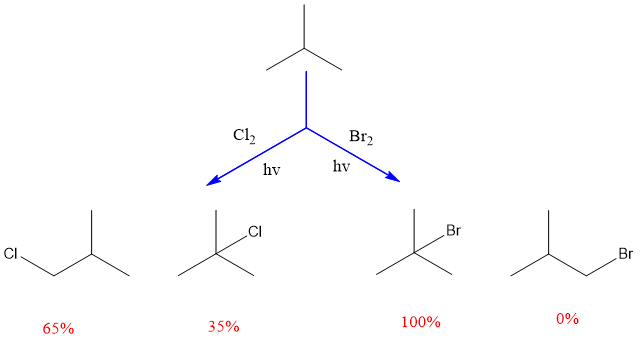
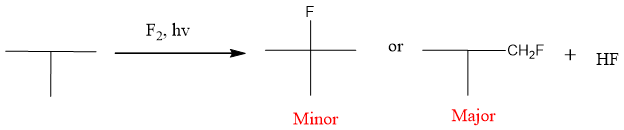
Only one product is formed with bromination, it is highly specific and significantly makes the product from the stable free radical. Among all halogens, fluorine is highly reactive and its reaction is highly exothermic and fast. Fluorination results in the formation of a mixture of products. The major product will be that which forms fastly.