
Orgoreview
Question Vault
Welcome to orgoreview question vault. We have 5000 more problems for you to solve.
A bottle of iodo-norbornane carboxylic acid contains a mixture of two diastereomers, A and B. You make the sodium salts of the carboxylic acids and only one of the isomers reacts to form a new compound, C. The other isomer does not react and remains the sodium salt.
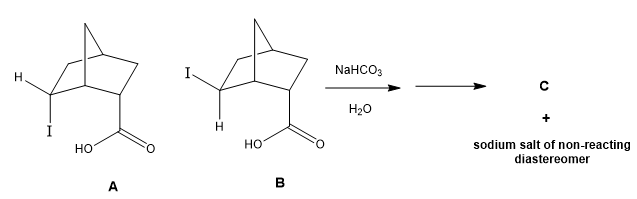
a. Draw the structure of compound C.
b. Is C the product of A or B?
c. Provide a one to two sentence mechanistic explanation for why one of the carboxylates reacts to form a new compound and the other does not

a. Draw the structure of compound C.
b. Is C the product of A or B?
c. Provide a one to two sentence mechanistic explanation for why one of the carboxylates reacts to form a new compound and the other does not