
Orgoreview
Question Vault
Welcome to orgoreview question vault. We have 5000 more problems for you to solve.
Vinyl alcohol and acetaldehyde both behave as weak acids. The ionization of vinyl alcohol and acetaldehyde both give the same conjugate base. Vinyl alcohol and acetaldehyde are constitutional isomers but vinyl alcohol is less stable than acetaldehyde.
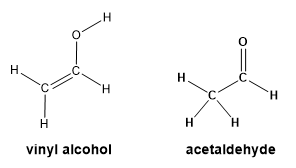 (a) Give an explanation for why these two acids give the same conjugate base.
(a) Give an explanation for why these two acids give the same conjugate base.
(b) Acetaldehyde has a pKa = 20. Predict whether vinyl alcohol is stronger or weaker acid than acetaldehyde and give a reason for your prediction.

(b) Acetaldehyde has a pKa = 20. Predict whether vinyl alcohol is stronger or weaker acid than acetaldehyde and give a reason for your prediction.