Orgoreview
Question Vault
Welcome to orgoreview question vault. We have 5000 more problems for you to solve.
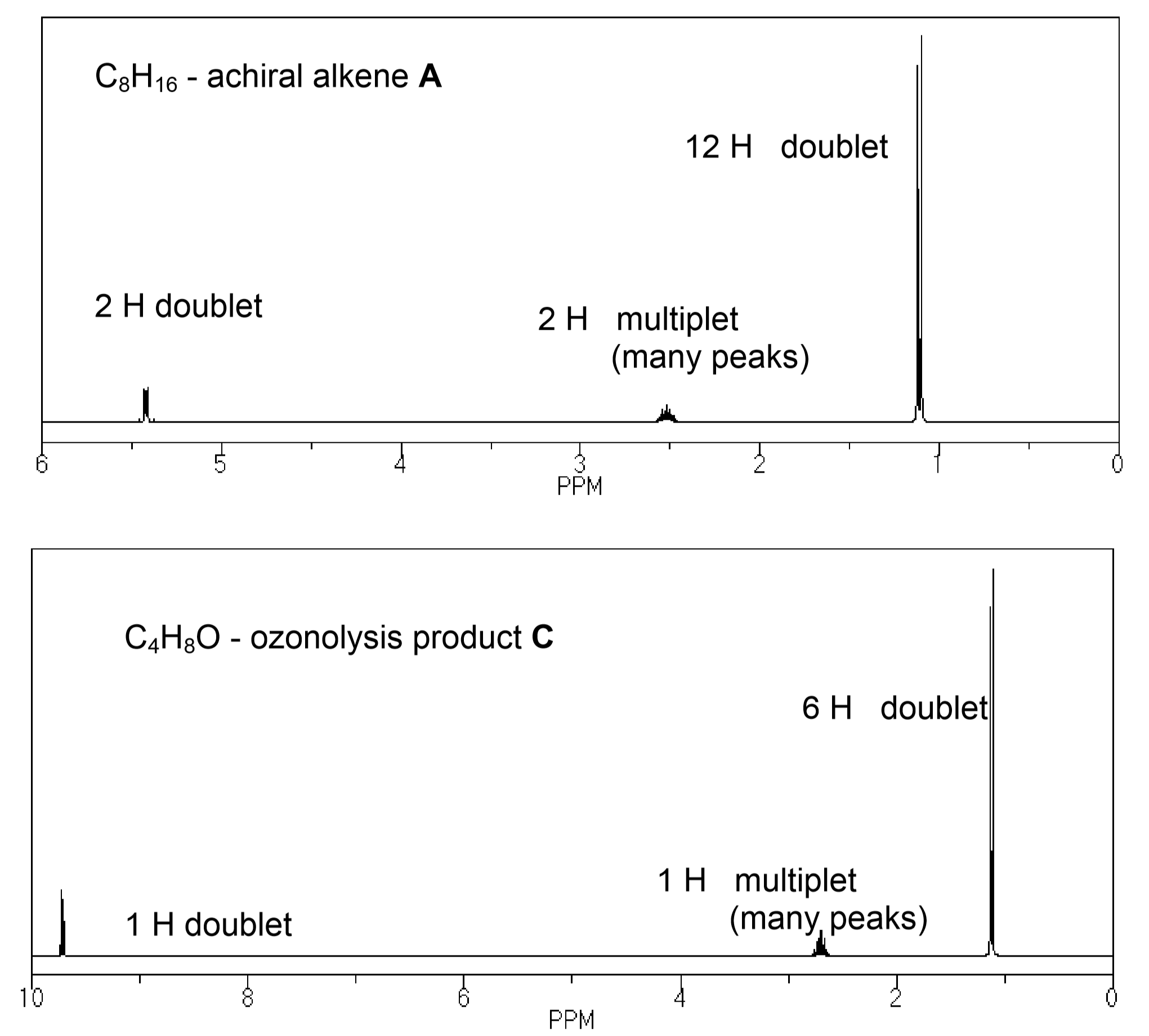
Compound A is an achiral alkene of formula C8H16. The NMR of A is shown below.
Oxymercuration of A gives a chiral alcohol, X, plus its enantiomer. Hydroboration gives the same products, X, as does the oxymercuration reaction.
Bromine addition in H2O gives compound, B, and its enantiomer.
Oxidation of A by OsO4 gives an achiral diol, Y.
Ozonolysis of A gives a single compound C, with formula C4H8O. C has the NMR shown below.

Draw structures for A, B, and C on your answer sheet. (You do not need to give structures for X and Y)