Orgoreview
Question Vault
Welcome to orgoreview question vault. We have 5000 more problems for you to solve.
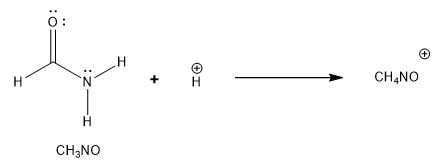
Formamide undergoes protonation on oxygen when treated with strong acid. Draw the two best contributing structures for this cation, identify the major contributor and, using the curved arrow formalism, show the bond making and bond breaking that occurs to interconvert these two structures.