
Orgoreview
Question Vault
Welcome to orgoreview question vault. We have 5000 more problems for you to solve.
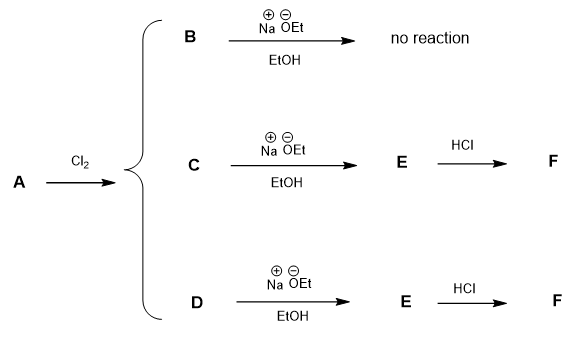
Propose structures for the lettered compounds based upon the data given below.
A =
B =
C =
D =
E =
F =
A =
B =
C =
D =
E =
F =
An alkane A (C6H14) reacts with chlorine to yield only three compounds (B, C, and D) with the formula C6H13Cl. Of these only C and D undergo dehydrohalogenation with sodium ethoxide in ethanol to produce an alkene. Moreover, C and D yield the same alkene E (C6H12). Treating E with HCl produces a compound F that is isomeric with B, C, and D.
