Sawhorse Projections
Sawhorse Projections:
Conformational isomerism:
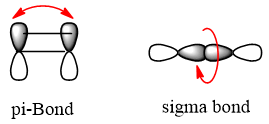
The rotation around double bond is impossible because it needs breaking of π bond and required high energy which is not available at room temperature. While sigma bonds are free to rotate, sigma bond is formed by head on overlap of orbitals which enable it to rotate in 3-dimensional space. Different rotations at different angles cause change in shape of molecule, and many different 3- dimensional arrangements of molecule are possible. These arrangements are called conformations, resulting via rotations about single bond and one particular conformation is called as conformational isomer or conformer.
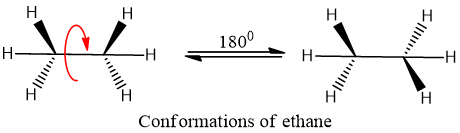
Conformational isomers or Conformations/Conformers:
The structures having same molecular formula but differ in arrangements of groups of atoms in 3-dimensional space associated to different energy, and can be interconverted by rotation around single bond.
Drawing conventions:
Different drawing conventions are used to visualize different conformations of molecule i-e Sawhorse, Natta, Newman projection. One convenient drawing convention which is often use to explain the stereochemistry of alkanes is Sawhorse projection.
Sawhorse Projection:
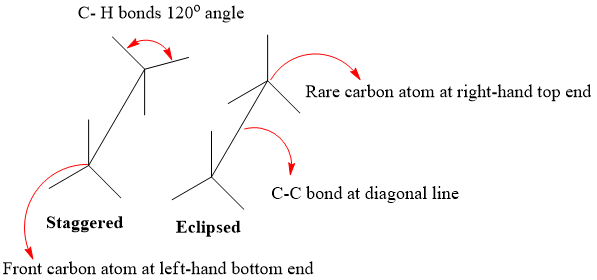
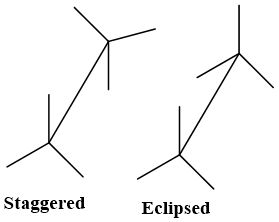
A drawing convention used to indicate the spatial arrangement of groups on adjacent carbon atoms. C- C bond when taken angular to plane of paper (diagonal line) an angular appearance of molecule results which is known as Sawhorse projection. Stereochemistry of Alkanes can be better explained by using Sawhorse projection, which visualizes conformation of a chemical bond from an oblique angle. It is named after its resemblance with carpenter’s sawhorse.
- Front Carbon atom (nearer to viewer) is located at left- hand bottom end.
- Rare carbon atom (away from viewer) is located at right-hand top end.
- C-C bond is present at diagonal line.
- C-H bonds are present at 1200 to each other.
Steps to draw Sawhorse Projection:
- 1. Selection of bond
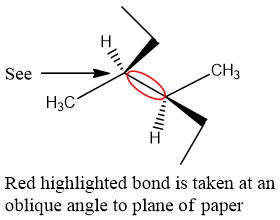
While looking at the alkane, you have to select a bond to be taken angular to plane of paper , and have to decide which ’C’ you want as front carbon atom and which as rare carbon atom.
- 2. Draw Sawhorse Skeleton
Red highlighted bond is drawn by a diagonal line
- 3. Attach side groups of front and back carbon atom
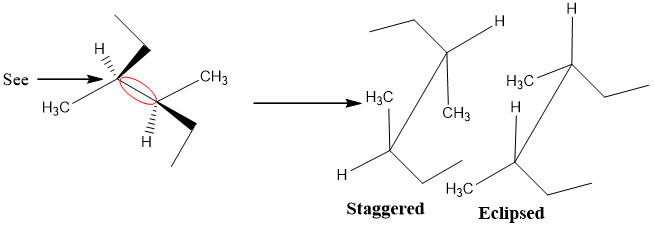
- 4. Other Conformations
Draw other conformations simply by rotating either back or front C atom.
Similarity with Newman:
- Both Sawhorse and Newman represent staggered and eclipsed conformations of molecule structure.
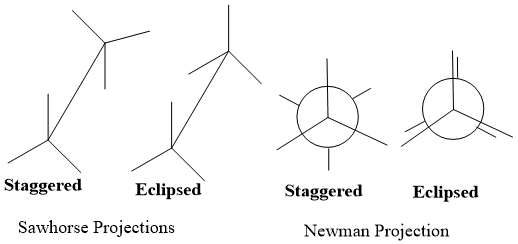
- Both are used to explain stereochemistry of alkanes.
Differences with Newman:
- Sawhorse projection is an angular representation of molecule while Newman is side on appearance of molecule.
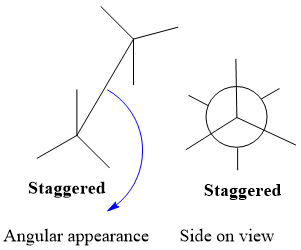
- C-C bond in Sawhorse is visible to viewer while hidden in Newman projection.
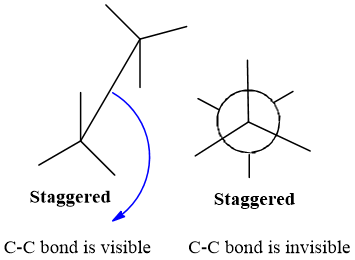
Importance of Sawhorse projection:
This projection is used to explain the interactions between groups of adjacent carbon atom. It is also useful to know enantiomeric and diastereomeric relation of two molecule. It helps to know if two molecules are mirror images or superimposable.
