Hinsberg Test
Hinsberg Test
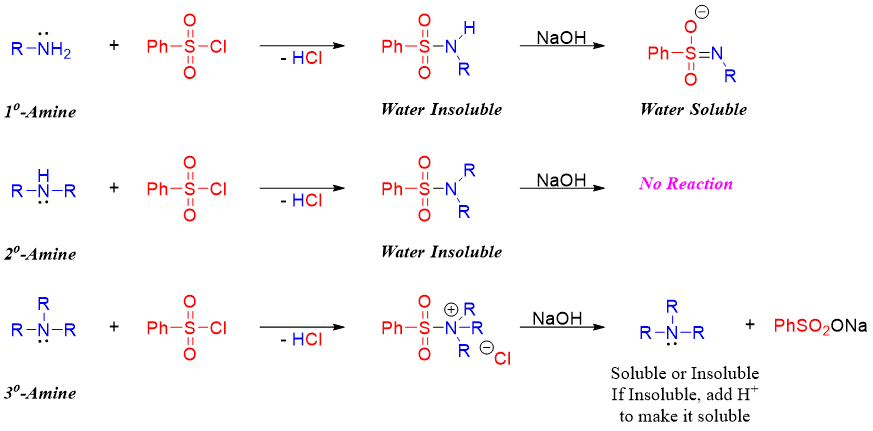
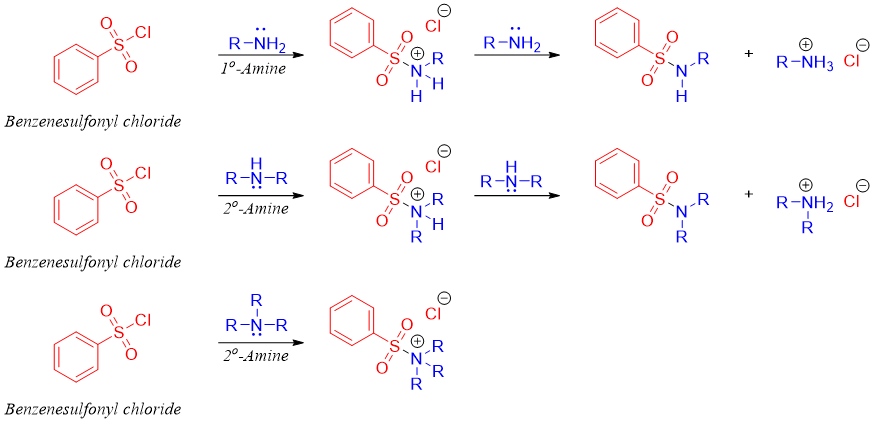
Hinsberg test is a reaction used to differentiate between primary, secondary, and tertiary amines. Amines being nucleophilic in nature can add to carbonyl compounds containing good leaving groups. Once the amine nitrogen is added to the carbonyl group the leaving group leaves resulting in nucleophilic acyl substitution reaction. In this test amines are reacted with benzene sulfonyl chloride in the presence of base like NaOH or KOH. An addition elimination reaction takes place forming benzene sulfonyl amine. Following scheme shows the reaction of all three classes of amines with benzene sulfonyl chloride.
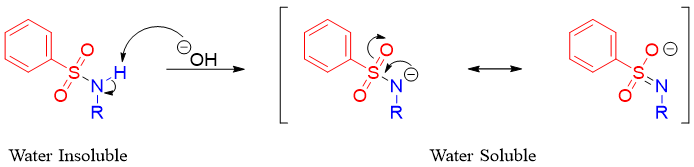
In Hinsberg test the reaction of primary amines gives mono-alkylated benzenesulfonyl amine as shown above. The mono-alkylated benzenesulfonyl amine is insoluble in water. The hydrogen attached to nitrogen atom is acidic in nature as the benzenesulfonyl group is electron withdrawing in nature. This acidic N-H proton is abstracted by base and forms water soluble anionic species shown below.
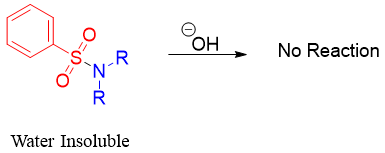
Reaction of secondary amines with benzenesulfonyl chloride produces dialkyl benzenesulfonyl amine. This molecule is insoluble in water. Unlike monoalkyl benzenesulfonyl amine, this molecule does not contain acidic proton. Therefore, this molecule remains insoluble in basic aqueous solution.
The reaction of ammonium cation (formed by tertiary amine and benzenesulfonyl chloride) upon reaction with base decomposes to form same tertiary amine and benzenesulfonate. If the tertiary amine is soluble in water, it will remain soluble. If the tertiary amine is insoluble in water, it will form an organic layer on the top of the water. This organic layer upon reaction with acid will dissolve in water.
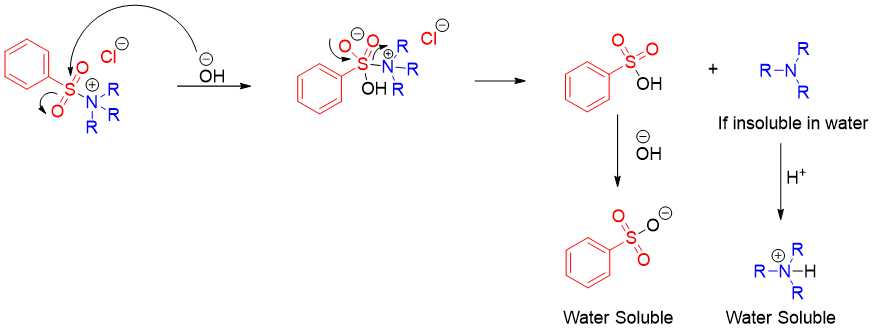
Following scheme summarizes the Hinsberg test for the identification of different classes of amines.