Potential Energy in Rings or Stability Trends of Rings
Potential energy and Stability Trend of Rings:
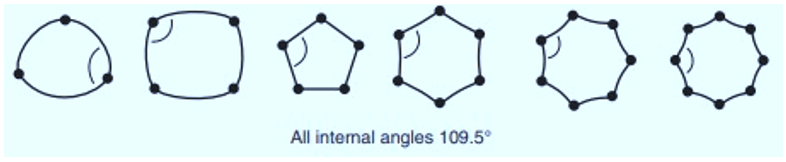
- In chemistry, rings are drawn by simple straight lines as they are planar, but this doesn’t happen in actual. Rings are not present in planar form. They infect exist in any other stable form. In fully saturated carbocyclic ring, each carbon atom is sp3 hybridized with bond angle 109.5o. This is the ideal angle for any atom forming bond via sp3 hybridization.
- If carbocyclic rings present in planar form as they usually drawn on paper, this ideal angle would not be satisfied. So, number of atoms in a ring are responsible for internal angle.
- Any deviation from normal tetrahedral angle causes un-stability to ring with some sort of strain in the molecule. Greater the deviation from ideal angle 109.5o, greater will be the strain in molecule, greater will be its potential energy, and smaller will be its stability.
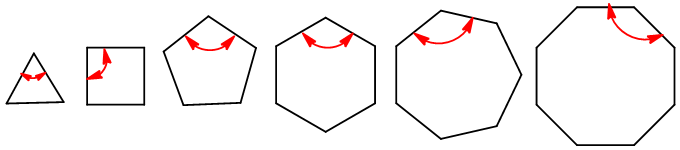
The rings with strain are forced to get the ideal angle 109.5o, to do so bonds in rings become curved. More the strain in the molecule more will be the curve bonds. Bonds in smaller rings being strained becomes curved outside and in larger rings being strained curved inside.
Baeyer’s strain theory; structure and stability of cycloalkanes:
A famous German organic chemist, Adolf Baeyer proposed a theory to explain the stability of smaller cycloalkane rings. He suggested that each carbon in cycloalkanes is sp3 hybridized. There must be 109.5o angle present in the ring. Any deviation from this normal tetrahedral angle would result in angular strain and cause increase in energy of ring. Greater the energy of ring, smaller will be the stability of ring.
Angle strain:
The deviation of internal angle from normal tetrahedral angle (109.5o) would result in internal strain of the ring, called angle strain.
Cyclopropane:
In cyclopropane each carbon undergoes sp3 hybridization. C-C bond is formed by the sp3-sp3 (head –on) overlap. Cyclopropane is equilateral triangle having 60o internal angle. This angle is showing deviation from normal tetrahedral angle by a value of 49.5o. This means bonds are pulled in by this value to maintain the ideal angle. Angle strain exists in cyclopropane, because the bonds in cyclopropane are banana shaped. This implies that an ineffective overlap occurs. A perfect head on overlap leads to 109.5o angle. The overlapping orbitals in cyclopropane are not pure sp3, they contain more p- character, overlap to form bond which is not purely σ in character.
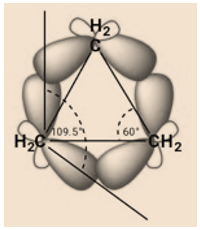
Potential energy Vs angular strain:
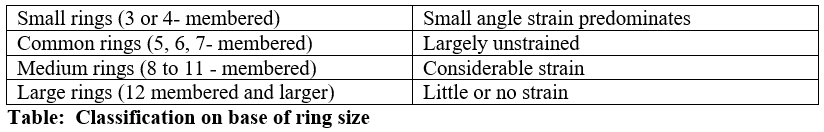
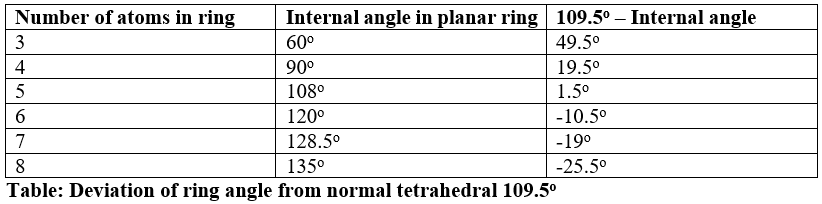
Greater the angle strain greater will be the energy of the ring. It can be seen from table angular strain is decreasing from 3 to 5 membered ring. This means cyclopropane is highly strained and have highest potential energy, easily undergo ring opening reaction.
Cyclopentane being least strained have lowest energy and don’t undergo ring opening reaction easily. Angular strain starts increasing again from cyclohexane to onwards.
Stability Vs angular strain:
Angular strain and stability are inversely linked. Greater the angle strain, greater will be energy and smaller will be the stability of ring. Cyclopropane is highly unstable. Stability increase up to cyclopentane then again starts decreasing on base of angular strain.
Limitations of Baeyer’s theory:
- Angular strain of cyclohexane is higher than cyclopentane, this implies that cyclohexane must be least stable than cyclopentane.
- But contrary to this prediction, cyclohexane and onwards rings don’t undergo ring opening reaction and they are least reactive.
- They resemble open chain alkanes in reactivity. Baeyer’s can’t explain this anomaly.
Stability of rings on base of heat of combustion:
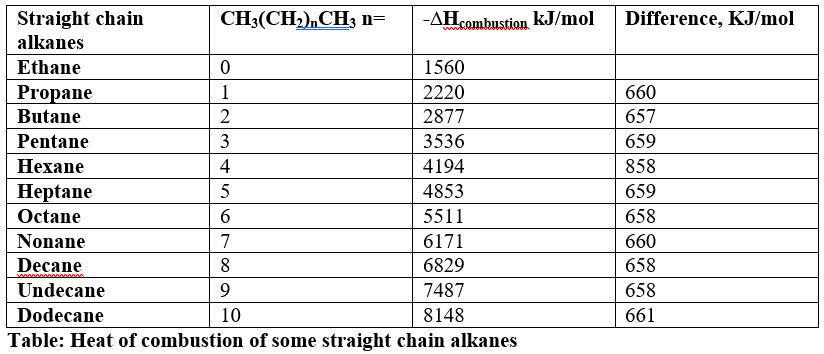
Angle strain in rings then measured by using heat of combustions. Consider the heat of combustion of some straight chain alkanes. It can be seen, there is a difference of around -660KJ/mol for two consecutive alkanes which means an amount of around -660 kJ/mol increase for each adding CH2. Heat of combustion of open chain alkanes is equal to -660 * CH2.
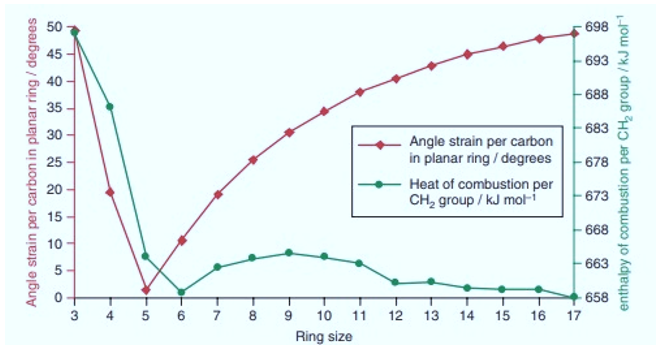
It can be seen in graph, Cyclopropane (n=3) has highest angle strain. The strain then begins to decrease by increase in number of atoms in ring, reaches a minimum for cycloheaxane not for cyclopentane as predicted by angle calculations. The angle strain again begins to increase but not rapidly as calculations suggested. The strain reaches a maximum at n=9 and again begins to decrease.
After n=14 the angle strain doesn’t increase according to ring size or angle calculations instead shows a roughly constant value.
The heat of combustion per CH2 for cyclohexane and larger cycloalkanes is around 658KJ/mol which is very close to straight chain alkanes. This indicates that Cycloalkanes and higher cycloalkanes are strain free.
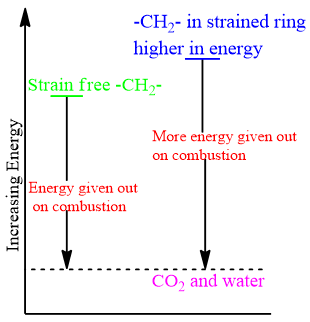
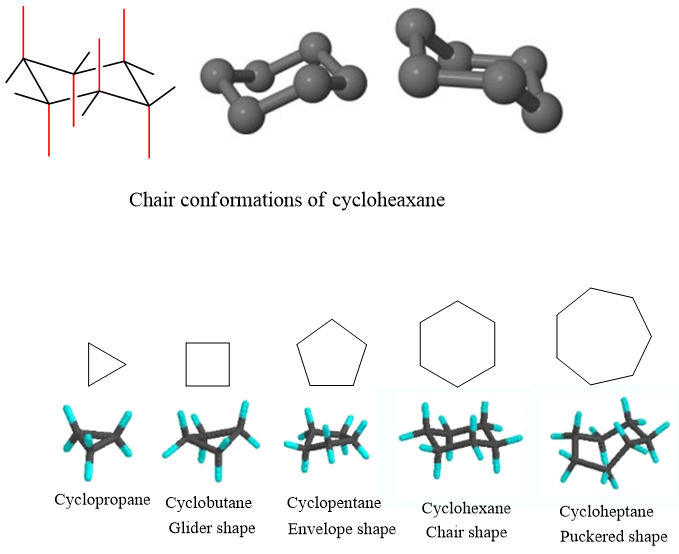
Puckered Forms:
In order to minimize the strain in the ring, Cycloalkanes like to exist in other cyclic conformations. These Cyclic forms are called puckered forms. In puckered forms internal angle is perfectly 109.5o.
Other types of strain present in rings:
- Torsional strain
- Steric strain
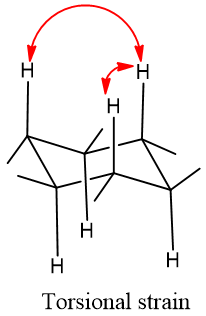
Torsional strain:
Repulsion between two adjacent hydrogen is called torsional strain.
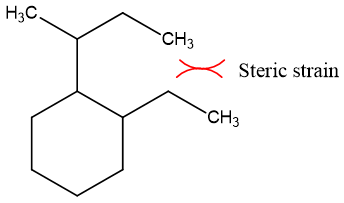
Steric strain:
Steric strain exists in trans-annualr rings. Steric strain is the repulsion between atoms present in close vicinity of each other.