Energy Calculations of Comformations
Staggered and eclipsed conformations and energy calculation of conformations:
The possibility of rotation about a sigma bond resulted in the formation of infinite number of spatial arrangement of atoms or (group of atoms) in a compound.
- Conformations of a compound are only differed slightly and can be inter-converted from one form to another form through bond rotation.
- Changing conformation does not mean breaking the bonds.
- Conformations of a compound are not considered as isomers they are all the same molecules.
- However, if different groups are attached to the atom about which rotation takes place then conformations of different energies can be formed depending on the steric hindrance of groups attached to the atom about which rotation takes place.
- The study of energetic of conformations of a compound is called as conformational analysis.
- Through conformational analysis the most favorable conformation can be detected.
- For doing the conformational analysis Newman projections of the compound are drawn.
- Two main conformations are possible for all the compounds; 1- conformation having highest energy is called as eclipsed conformation. 2- Conformation having lowest energy is called as staggered conformation.
- The difference of energy is due to the presence of rotational barrier which depends on the steric hindrance of groups attach to the carbon about which bonds are rotated. The more the chance of two bulkier groups to come together the more energy is required to do that rotation.
- The simplest example for the conformational analysis is the analysis of ethane;
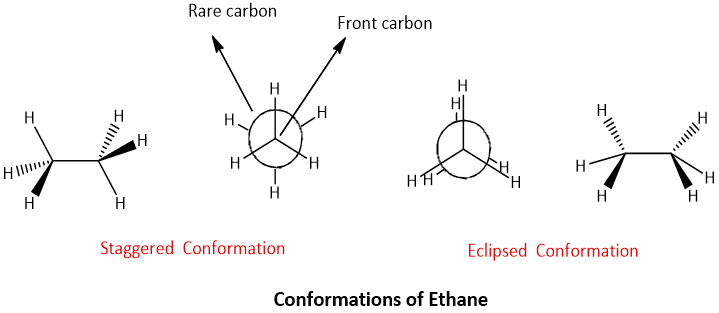
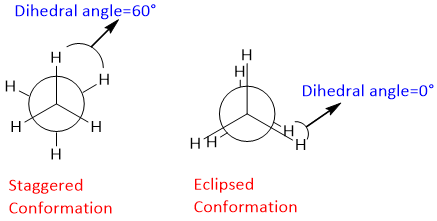
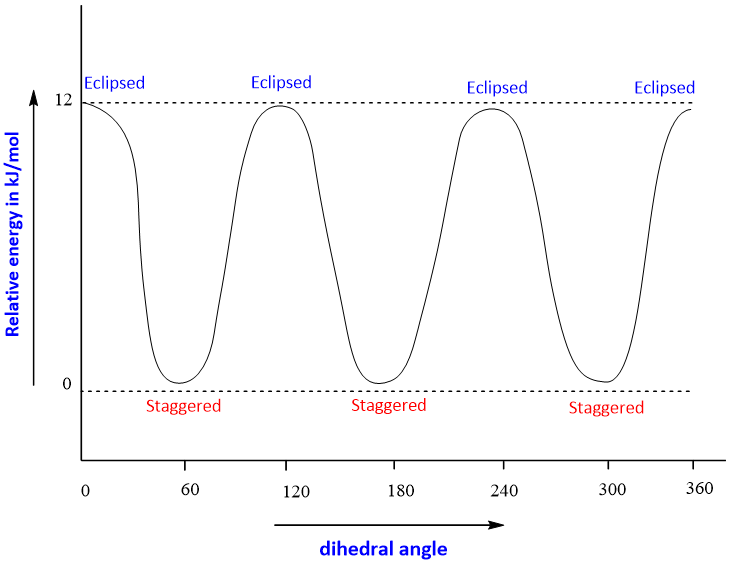
The difference of energy between the staggered and eclipsed conformation of ethane is about 12kJ/mol. Dihedral angle; the angle between the C-H bond on the front carbon and C-H bond on the rare (farthest) carbon is called as dihedral angle. It is also called as torsional angle due to presence of torsional strain (steric hindrance) between two hydrogen atoms.
In case of staggered conformation, the torsional strain, and for eclipsed conformation.
The energy profile diagram for eclipsed and staggered conformation is shown as;
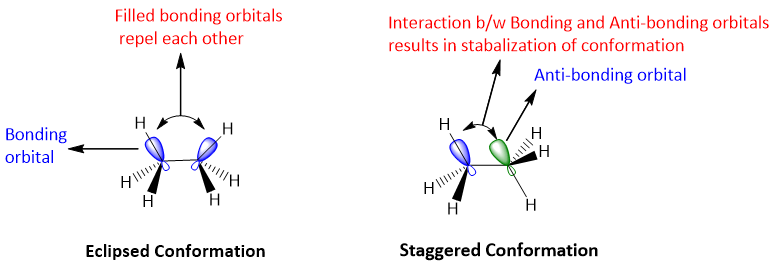
Reason for stability of staggered conformation:
The bonding electrons repel each other because of similar charge on electrons. When the bonding electrons come parallel to each other, repulsion between them increases. This results in the increase in energy of the system which causes instability. Another reason for the stability of staggered conformation is the development of interactions between C-H bonding orbital and sigma-antibonding orbital of other carbon. This interaction creates stability, and occurs when both bonding and anti-bonding orbitals are parallel to each other i.e. the two bonding orbitals are apart from each other.
Conformational Analysis of Propane:
Propane has the formula of CH3CH2CH3. Propane has the same conformations as that of ethane. But the energy barrier for rotation is 14kJ/mol for propane, this is due to the presence of one extra CH3 group in propane which is absent in ethane.
The conformations for propane are shown below;
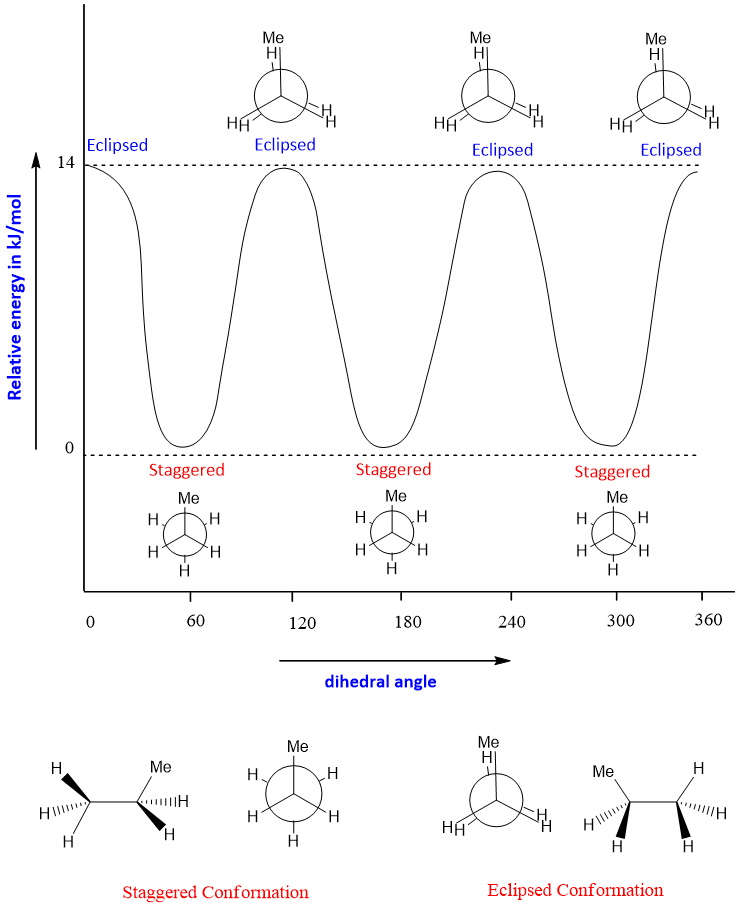
While performing conformational analysis one of the carbon (either front or rare carbon) is kept fixed and the bond on the other carbon are rotated at an angle of 60 and rotations are done till 360 angle.
Conformational Analysis of butane:
The energy barrier for butane is more than ethane and propane, 20kJ/mol. The conformational analysis of butane is shown below;
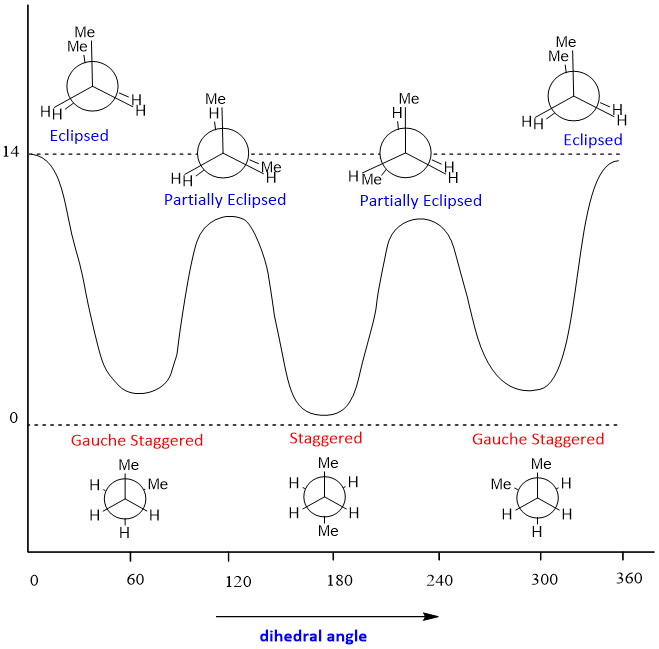
The conformational analysis of butane showed that there are two type staggered conformations, Gauche staggered and completely staggered. Two types of eclipsed conformations partially eclipsed and fully eclipsed. From all conformations, the lowest energy and most stable conformation is completely staggered conformation (anti-periplanar). The highest energy and least stable conformation is fully eclipsed conformation (syn-periplanar).
In fully eclipsed conformation both methyl groups are at an angle of zero degrees, there is high steric strain is present between these two methyl groups, in partially eclipsed conformation, one hydrogen is present at zero degree with other hydrogen. These two hydrogens offer torsional strain which is less effective than steric strain. Because of this reason fully eclipsed conformation has more energy than partially eclipsed conformation. The relative energy of conformations is given as;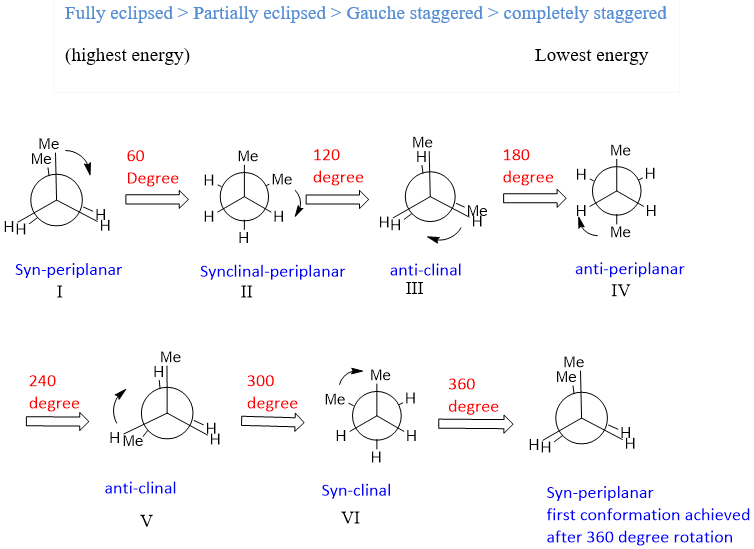
It is clear that conformations refer to all arrangements possible for the compound but conformers are those conformations that are stereo-isomers.
